Exploring Integrin α5β1 as a Potential Therapeutic Target for Pulmonary Arterial Hypertension: Insights From Comprehensive Multicenter Preclinical Studies
- PMID: 39829438
- PMCID: PMC12011439
- DOI: 10.1161/CIRCULATIONAHA.124.070693
Exploring Integrin α5β1 as a Potential Therapeutic Target for Pulmonary Arterial Hypertension: Insights From Comprehensive Multicenter Preclinical Studies
Abstract
Background: Pulmonary arterial hypertension (PAH) is characterized by obliterative vascular remodeling of the small pulmonary arteries (PAs) and progressive increase in pulmonary vascular resistance leading to right ventricular failure. Although several drugs are approved for the treatment of PAH, mortality rates remain high. Accumulating evidence supports a pathological function of integrins in vessel remodeling, which are gaining renewed interest as drug targets. However, their role in PAH remains largely unexplored.
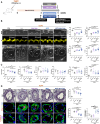
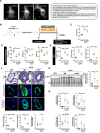
Methods: The expression of the RGD (arginylglycylaspartic acid)-binding integrin α5β1 was assessed in PAs, PA smooth muscle cells, and PA endothelial cells from patients with PAH and controls using NanoString, immunoblotting, and Mesoscale Discovery assays. RNA sequencing was conducted to identify gene networks regulated by α5β1 inhibition in PAH PA smooth muscle cells. The therapeutic efficacy of α5β1 inhibition was evaluated using a novel small molecule inhibitor and selective neutralizing antibodies in Sugen/hypoxia and monocrotaline rat models, with validation by an external contract research organization. Comparisons were made against standard-of-care therapies (ie, macitentan, tadalafil) and sotatercept and efficacy was assessed using echocardiographic, hemodynamic, and histological assessments. Ex vivo studies using human precision-cut lung slices were performed to further assess the effects of α5β1 inhibition on pulmonary vascular remodeling.
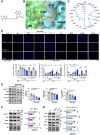
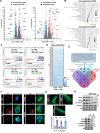
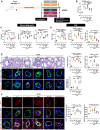
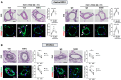
Results: We found that the arginine-glycine-aspartate RGD-binding integrin α5β1 is upregulated in PA endothelial cells and PA smooth muscle cells from patients with PAH and remodeled PAs from animal models. Blockade of the integrin α5β1 or depletion of the α5 subunit downregulated FOXM1 (forkhead box protein M1)-regulated gene networks, resulting in mitotic defects and inhibition of the pro-proliferative and apoptosis-resistant phenotype of PAH cells. We demonstrated that α5β1 integrin blockade safely attenuates pulmonary vascular remodeling and improves hemodynamics and right ventricular function and matched or exceeded the efficacy of standard of care and sotatercept in multiple preclinical models. Ex vivo studies further validated its potential in reversing advanced remodeling in human precision-cut lung slices.
Conclusions: These findings establish α5β1 integrin as a pivotal driver of PAH pathology and we propose its inhibition as a novel, safe, and effective therapeutic strategy for PAH.
Keywords: extracellular matrix; heart ventricles; hypertension, pulmonary; integrins; models, animal; vascular remodeling.
Conflict of interest statement
This study was partially funded by Morphic Therapeutic.
Figures


Update of
-
Exploring Integrin α5β1 as a Potential Therapeutic Target for Pulmonary Arterial Hypertension: Insights from Comprehensive Multicenter Preclinical Studies.bioRxiv [Preprint]. 2024 Jun 1:2024.05.27.596052. doi: 10.1101/2024.05.27.596052. bioRxiv. 2024. Update in: Circulation. 2025 Apr 22;151(16):1162-1183. doi: 10.1161/CIRCULATIONAHA.124.070693. PMID: 38854025 Free PMC article. Updated. Preprint.
Comment in
-
Integrin Targeting Therapies in Pulmonary Arterial Hypertension: A Roadmap for Traversing the Translational Valley of Death?Circulation. 2025 Apr 22;151(16):1184-1186. doi: 10.1161/CIRCULATIONAHA.125.074059. Epub 2025 Apr 21. Circulation. 2025. PMID: 40258053 No abstract available.
References
-
- Humbert M, Guignabert C, Bonnet S, Dorfmuller P, Klinger JR, Nicolls MR, Olschewski AJ, Pullamsetti SS, Schermuly RT, Stenmark KR, et al. . Pathology and pathobiology of pulmonary hypertension: state of the art and research perspectives. Eur Respir J. 2019;53:1801887. doi: 10.1183/13993003.01887-2018 - PMC - PubMed
-
- Pullamsetti SS, Savai R, Seeger W, Goncharova EA. Translational advances in the field of pulmonary hypertension: from cancer biology to new pulmonary arterial hypertension therapeutics: targeting cell growth and proliferation signaling hubs. Am J Respir Crit Care Med. 2017;195:425–437. doi: 10.1164/rccm.201606-1226PP - PMC - PubMed
-
- Humbert M, Sitbon O, Guignabert C, Savale L, Boucly A, Gallant-Dewavrin M, McLaughlin V, Hoeper MM, Weatherald J. Treatment of pulmonary arterial hypertension: recent progress and a look to the future. Lancet Respir Med. 2023;11:804–819. doi: 10.1016/S2213-2600(23)00264-3 - PubMed
-
- Lajoie AC, Lauziere G, Lega JC, Lacasse Y, Martin S, Simard S, Bonnet S, Provencher S. Combination therapy versus monotherapy for pulmonary arterial hypertension: a meta-analysis. Lancet Respir Med. 2016;4:291–305. doi: 10.1016/S2213-2600(16)00027-8 - PubMed
-
- Hoeper MM, Badesch DB, Ghofrani HA, Gibbs JSR, Gomberg-Maitland M, McLaughlin VV, Preston IR, Souza R, Waxman AB, Grunig E, et al. ; STELLAR Trial Investigators. Phase 3 trial of sotatercept for treatment of pulmonary arterial hypertension. N Engl J Med. 2023;388:1478–1490. doi: 10.1056/NEJMoa2213558 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

