This is a preprint.
Butyrolactol A potentiates caspofungin efficacy against resistant fungi via phospholipid flippase inhibition
- PMID: 39829750
- PMCID: PMC11741340
- DOI: 10.1101/2025.01.06.630955
Butyrolactol A potentiates caspofungin efficacy against resistant fungi via phospholipid flippase inhibition
Abstract
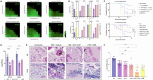
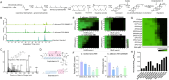
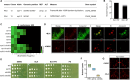
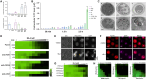
Fungal infections cause millions of deaths annually and are challenging to treat due to limited therapeutic options and rising resistance. Cryptococci are intrinsically resistant to the latest generation of antifungals, echinocandins, while Candida auris, a notorious global threat, is also increasingly resistant. We performed a natural product extract screen to rescue caspofungin fungicidal activity against Cryptococcus neoformans H99 and identified butyrolactol A, which restores echinocandin efficacy against resistant fungal pathogens, including multidrug-resistant C. auris. Mode of action studies revealed that butyrolactol A inhibits the phospholipid flippase Apt1-Cdc50, blocking phospholipid transport. Cryo-electron microscopy analysis of the Apt1•butyrolactol A complex revealed that the flippase is trapped in a dead-end state. Apt1 inhibition disrupts membrane asymmetry, vesicular trafficking, and cytoskeletal organization, thereby enhancing echinocandin uptake and potency. This study identifies lipid flippases as promising antifungal targets and demonstrates the potential of revisiting natural products to expand the antifungal arsenal and combat resistance.
Keywords: Apt1–Cdc50; Candida auris; Cryptococcus; antifungal; echinocandin; flippase; fungal resistance; natural product; synergy.
Conflict of interest statement
DECLARATION OF INTERESTS L.E.C. is a co-founder and shareholder in Bright Angel Therapeutics, a platform company for the development of novel antifungal therapeutics. E.D.B is the CEO and L.E.C. and G.D.W. are Science Advisors for Kapoose Creek Bio, a company that harnesses the therapeutic potential of fungi. All other authors have no competing interests to declare.
Figures


References
-
- Brown G.D., Denning D.W., Gow N.A.R., Levitz S.M., Netea M.G., and White T.C. (2012). Hidden Killers: Human Fungal Infections. Sci. Transl. Med. 4, 165rv13–165rv13. 10.1126/scitranslmed.3004404. - DOI
-
- Bongomin F., Gago S., Oladele R., and Denning D. (2017). Global and Multi-National Prevalence of Fungal Diseases—Estimate Precision. J. Fungi 3, 57. 10.3390/jof3040057. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
