This is a preprint.
ChromBPNet: bias factorized, base-resolution deep learning models of chromatin accessibility reveal cis-regulatory sequence syntax, transcription factor footprints and regulatory variants
- PMID: 39829783
- PMCID: PMC11741299
- DOI: 10.1101/2024.12.25.630221
ChromBPNet: bias factorized, base-resolution deep learning models of chromatin accessibility reveal cis-regulatory sequence syntax, transcription factor footprints and regulatory variants
Abstract
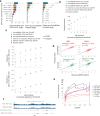
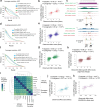
Despite extensive mapping of cis-regulatory elements (cREs) across cellular contexts with chromatin accessibility assays, the sequence syntax and genetic variants that regulate transcription factor (TF) binding and chromatin accessibility at context-specific cREs remain elusive. We introduce ChromBPNet, a deep learning DNA sequence model of base-resolution accessibility profiles that detects, learns and deconvolves assay-specific enzyme biases from regulatory sequence determinants of accessibility, enabling robust discovery of compact TF motif lexicons, cooperative motif syntax and precision footprints across assays and sequencing depths. Extensive benchmarks show that ChromBPNet, despite its lightweight design, is competitive with much larger contemporary models at predicting variant effects on chromatin accessibility, pioneer TF binding and reporter activity across assays, cell contexts and ancestry, while providing interpretation of disrupted regulatory syntax. ChromBPNet also helps prioritize and interpret regulatory variants that influence complex traits and rare diseases, thereby providing a powerful lens to decode regulatory DNA and genetic variation.
Conflict of interest statement
Competing interests: A.S. was a consultant for MyoKardia. W.J.G. is a consultant and equity holder for 10x Genomics, Nvidia, Guardant Health, Quantapore, and Ultima Genomics, and cofounder of Protillion Biosciences, and is named on patents describing ATAC-seq.. A.S is an employee of Insitro. S.N is an employee of Genentech. A.S.K is an employee of Ultima Genomics. V.R is an employee of Rockefeller University. A.K. is on the scientific advisory board of SerImmune, TensorBio, AINovo, is a consultant with Arcardia Science, Inari, Precede Biosciences, was a consultant with Illumina and PatchBio and has a financial stake in DeepGenomics, Immunai and Freenome.
Figures















References
-
- Wasserman W. W. & Sandelin A. Applied bioinformatics for the identification of regulatory elements. Nat. Rev. Genet. 5, 276–287 (2004). - PubMed
-
- Morgunova E. & Taipale J. Structural perspective of cooperative transcription factor binding. Curr Opin Struct Biol 47, 1–8 (2017). - PubMed
-
- Isbel L., Grand R. S. & Schübeler D. Generating specificity in genome regulation through transcription factor sensitivity to chromatin. Nat. Rev. Genet. 23, 728–740 (2022). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
