This is a preprint.
Aging and injury drive neuronal senescence in the dorsal root ganglia
- PMID: 39829815
- PMCID: PMC11741248
- DOI: 10.1101/2024.01.20.576299
Aging and injury drive neuronal senescence in the dorsal root ganglia
Update in
-
Aging and injury drive neuronal senescence in the dorsal root ganglia.Nat Neurosci. 2025 May;28(5):985-997. doi: 10.1038/s41593-025-01954-x. Epub 2025 May 14. Nat Neurosci. 2025. PMID: 40369367 Free PMC article.
Abstract
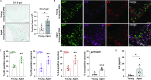
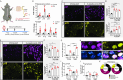
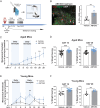
Aging negatively impacts central nervous system function; however, the cellular impact of aging in the peripheral nervous system remains poorly understood. Aged individuals are more likely to experience increased pain and slower recovery after trauma. Such injury can damage vulnerable peripheral axons of dorsal root ganglion (DRG) neurons resulting in somatosensory dysfunction. One cellular mechanism common to both aging and injury is cellular senescence, a complex cell state that can contribute to the aged pro-inflammatory environment. We uncovered, for the first time, DRG neuron senescence in the context of aging and pain-inducing peripheral nerve injury in young and aged mice. Aged DRG neurons displayed multiple markers of senescence (SA-β-gal, p21, p16, IL6) when compared to young DRG neurons. Peripheral nerve injury triggered a further accumulation of senescent DRG neurons over time post-injury in young and aged DRG. These senescent neurons were dynamic and heterogeneous in their expression of senescence markers, p16, p21, and senescence-associated secretory phenotype (SASP) expression of IL6, which was influenced by age. An electrophysiological characterization of senescence marker-expressing neurons revealed high-firing and nociceptor-like phenotypes within these populations. In addition, we observed improvement in nociceptive behaviors in young and aged nerve-injured mice after treatment with a senolytic agent that eliminates senescent cells. Finally, we confirmed in human post-mortem DRG samples that neuronal senescence is present and increases with age. Overall, we describe a susceptibility of the peripheral nervous system to neuronal senescence with age or injury that may be a targetable mechanism to treat sensory dysfunction, such as chronic pain, particularly in aged populations.
Keywords: Neuron; chronic pain; human; mouse; spared nerve injury.
Conflict of interest statement
COMPETING FINANCIAL INTERESTS The authors have declared that no conflict of interest exists
Figures




References
-
- Millecamps M, Shi XQ, Piltonen M, Echeverry S, Diatchenko L, Zhang J, et al. The geriatric pain experience in mice: intact cutaneous thresholds but altered responses to tonic and chronic pain. Neurobiol Aging. 2020;89:1–11. - PubMed
-
- Canta A, Chiorazzi A, Carozzi VA, Meregalli C, Oggioni N, Bossi M, et al. Age-related changes in the function and structure of the peripheral sensory pathway in mice. Neurobiol Aging. 2016;45:136–48. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
