This is a preprint.
Regionalized regulation of actomyosin organization influences cardiomyocyte cell shape changes during chamber curvature formation
- PMID: 39829878
- PMCID: PMC11741281
- DOI: 10.1101/2025.01.07.631779
Regionalized regulation of actomyosin organization influences cardiomyocyte cell shape changes during chamber curvature formation
Abstract
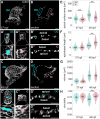
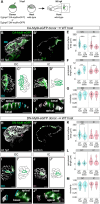
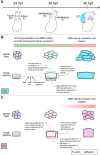
Cardiac chambers emerge from a heart tube that balloons and bends to create expanded ventricular and atrial structures, each containing a convex outer curvature (OC) and a recessed inner curvature (IC). The cellular and molecular mechanisms underlying the formation of these characteristic curvatures remain poorly understood. Here, we demonstrate in zebrafish that the initially similar populations of OC and IC ventricular cardiomyocytes diverge in the organization of their actomyosin cytoskeleton and subsequently acquire distinct OC and IC cell shapes. Altering actomyosin dynamics hinders cell shape changes in the OC, and mosaic analyses indicate that actomyosin regulates cardiomyocyte shape in a cell-autonomous manner. Additionally, both biomechanical cues and the transcription factor Tbx5a influence the basal enrichment of actomyosin and squamous cell morphologies in the OC. Together, our findings demonstrate that intrinsic and extrinsic factors intersect to control actomyosin organization in OC cardiomyocytes, which in turn promotes the cell shape changes that accompany curvature morphogenesis.
Conflict of interest statement
Competing interests Authors declare that they have no competing interests.
Figures





References
-
- Aphalo P. (2024). ggpmisc: Miscellaneous Extensions to “ggplot2” [Computer software]. https://CRAN.R-project.org/package=ggpmisc
-
- Auman H. J., Fernandes I. H., Berríos-Otero C. A., Colombo S., & Yelon D. (2024). Zebrafish smarcc1a mutants reveal requirements for BAF chromatin remodeling complexes in distinguishing the atrioventricular canal from the cardiac chambers. Developmental Dynamics, 253(1), 157–172. 10.1002/dvdy.595 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
