This is a preprint.
The Q226L mutation can convert a highly pathogenic H5 2.3.4.4e virus to bind human-type receptors
- PMID: 39829928
- PMCID: PMC11741302
- DOI: 10.1101/2025.01.10.632119
The Q226L mutation can convert a highly pathogenic H5 2.3.4.4e virus to bind human-type receptors
Update in
-
The Q226L mutation can convert a highly pathogenic H5 2.3.4.4e virus to bind human-type receptors.Proc Natl Acad Sci U S A. 2025 Apr 22;122(16):e2419800122. doi: 10.1073/pnas.2419800122. Epub 2025 Apr 15. Proc Natl Acad Sci U S A. 2025. PMID: 40232794
Abstract
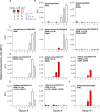
H5Nx viruses continue to wreak havoc in avian and mammalian species worldwide. The virus distinguishes itself by the ability to replicate to high titers and transmit efficiently in a wide variety of hosts in diverse climatic environments. Fortunately, transmission to and between humans is scarce. Yet, if such an event were to occur, it could spark a pandemic as humans are immunologically naïve to H5 viruses. A significant determinant of transmission to and between humans is the ability of the influenza A virus hemagglutinin (HA) protein to shift from an avian-type to a human-type receptor specificity. Here, we demonstrate that a 2016 2.3.4.4e virus HA can convert to human-type receptor binding via a single Q226L mutation, in contrast to a cleavage-modified 2016 2.3.4.4b virus HA. Using glycan arrays, x-ray structural analyses, tissue- and direct glycan binding, we show that L133aΔ and 227Q are vital for this phenotype. Thus, whereas the 2.3.4.4e virus HA only needs a single amino acid mutation, the modified 2.3.4.4b HA was not easily converted to human-type receptor specificity.
Figures


References
-
- Xu X., Subbarao, Cox N. J., Guo Y., Genetic characterization of the pathogenic influenza A/Goose/Guangdong/1/96 (H5N1) virus: similarity of its hemagglutinin gene to those of H5N1 viruses from the 1997 outbreaks in Hong Kong. Virology 261, 15–19 (1999). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
