Biologics as well as inhaled anti-asthmatic therapy achieve clinical remission: Evidence from the Severe Asthma Network in Italy (SANI)
- PMID: 39829953
- PMCID: PMC11741032
- DOI: 10.1016/j.waojou.2024.101016
Biologics as well as inhaled anti-asthmatic therapy achieve clinical remission: Evidence from the Severe Asthma Network in Italy (SANI)
Abstract
Background: This study aimed to evaluate the impact of severe asthma (SA) treatments after 12 months in achieving clinical remission (CR) within the context of the Severe Asthma Network in Italy (SANI) using the recent SANI definition of CR on treatment.
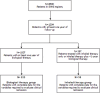
Methods: CR has been defined by SANI as complete, partial, and no CR. Complete CR is defined by the absence of oral corticosteroids (OCS), no symptoms, no exacerbations, and stable lung function, and partial CR requires the absence of OCS and the fulfillment of 2 out of the other 3 criteria. Patients who do not meet the previous criteria do not reach CR.
Results: After 12 months of treatment, 283 patients were selected to evaluate the effectiveness of biologics (225 patients) and inhaled therapy (58 patients) in achieving CR. Among patients treated with biologic agents, 45.8% reached complete CR, 23.1% partial CR, and 31.1% no CR. Differences in CR achievement according to type of biologic agent administered were observed. Interesting results were found when assessing the inhaled therapy (ICS/LABA/LAMA and no biologics) effectiveness: 34.5% patients reached complete CR, 34.5% partial CR, and 31.0% did not reach CR. This finding is noteworthy since it further supports the efficacy of inhaled treatment in certain SA patients and highlights the relevance of using CR as a modern outcome of SA treatments. Chronic rhinosinusitis with nasal polyps (CRSwNP) comorbidity was associated, though not significantly, with CR achievement in patients treated with biologics. Asthma Control Test (ACT) and Asthma Control Questionnaire (ACQ) scores significantly impacted CR (p = 0.003 and p = 0.027, respectively), while biomarkers, namely IgE, blood eosinophils, or fractional exhaled nitric oxide (FeNO), were not associated with CR achievement.
Conclusions: This study confirmed the effectiveness of biologics in reaching CR and demonstrated also inhaled therapies able to achieve CR. These innovative findings should encourage post hoc analysis of randomized clinical trials or even retrospective analysis of SA patient cohorts to evaluate CR with different inhaled treatments and further define the populations eligible for each treatment.
Trial registration: ClinicalTrials.gov ID: NCT06625216; Central Ethics Committee: Comitato Etico Area Vasta Nord-Ovest Toscana (study number 1245/2016, protocol number:73714).
Keywords: Biologics; Clinical remission; Inhaled therapy; Registry; Severe asthma.
© 2024 The Authors.
Conflict of interest statement
GWC reports research or clinical trials grants paid to his Institution from Menarini, AstraZeneca,GSK, Sanofi Genzyme and fees for lectures or advisory board participation from Menarini, AstraZeneca, CellTrion, Chiesi, Faes Farma, Firma, Genentech, Guidotti-Malesci, GSK, HAL Allergy, Innovacaremd, Novartis, OM-Pharma, Red Maple, Sanofi-Aventis, Sanofi-Genzyme, Stallergenes-Greer and Uriach Pharma. FB reports grants or contracts from AstraZeneca, Chiesi, and Insmed; consulting fees from Menarini; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from AstraZeneca, Chiesi, GSK, Guidotti, Grifols, Insmed, Menarini, OM Pharma, Pfizer, Sanofi, Viatris, Vertex, and Zambon. PP reports grants and/or personal fees from AstraZeneca, Chiesi Farmaceutici, GSK, Guidotti, and Sanofi outside the submitted work. EH reports grants and/or personal fees from AstraZeneca, GSK, Sanofi, Regeneron, Novartis, Chiesi, Stallergenes-Greer, Bosch, Celltrion-Healthcare, Almirall outside the submitted work. CC, CO, IS, and VB do not have any conflict of interest to declare.
Figures
References
-
- Hashmi MF, Cataletto ME. Asthma. https://www.ncbi.nlm.nih.gov/books/NBK430901/.
-
- GINA-2024-Strategy-Report-24_05_22_WMS.
Associated data
LinkOut - more resources
Full Text Sources
Medical