Prodrugs and their activation mechanisms for brain drug delivery
- PMID: 39829971
- PMCID: PMC11740913
- DOI: 10.1039/d4md00788c
Prodrugs and their activation mechanisms for brain drug delivery
Abstract
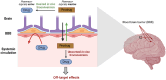
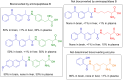
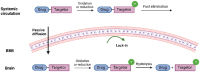
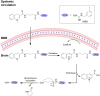
Prodrugs are masked drugs that first become pharmacologically active after undergoing a structural change in vivo. They are designed to improve physicochemical/biopharmaceutical drug properties and increase site specificity. The prodrug approach is important when developing brain-targeting drugs due to the presence of the brain barriers that seriously limit the brain entry of highly polar, multifunctional drug entities. While several excellent reviews summarize the structural modifications facilitating transport across the brain barriers, a summary of mechanisms used for the activation of the prodrug in the brain is missing. Given the high need for innovative discoveries in brain drug development, we here review the most important tools being developed since 2000 for CNS prodrug activation.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures












Similar articles
-
Prodrug approaches for CNS delivery.AAPS J. 2008;10(1):92-102. doi: 10.1208/s12248-008-9009-8. Epub 2008 Feb 5. AAPS J. 2008. PMID: 18446509 Free PMC article. Review.
-
Prodrugs: a challenge for the drug development.Pharmacol Rep. 2013;65(1):1-14. doi: 10.1016/s1734-1140(13)70959-9. Pharmacol Rep. 2013. PMID: 23563019 Review.
-
Prodrugs for Improved Drug Delivery: Lessons Learned from Recently Developed and Marketed Products.Pharmaceutics. 2020 Oct 29;12(11):1031. doi: 10.3390/pharmaceutics12111031. Pharmaceutics. 2020. PMID: 33137942 Free PMC article. Review.
-
Increasing oral absorption of polar neuraminidase inhibitors: a prodrug transporter approach applied to oseltamivir analogue.Mol Pharm. 2013 Feb 4;10(2):512-22. doi: 10.1021/mp300564v. Epub 2013 Jan 4. Mol Pharm. 2013. PMID: 23244438 Free PMC article.
-
Progress in drug delivery to the central nervous system by the prodrug approach.Molecules. 2008 May 1;13(5):1035-65. doi: 10.3390/molecules13051035. Molecules. 2008. PMID: 18560328 Free PMC article. Review.
Cited by
-
Prodrugs 2-(Phosphonomethyl)pentanedioic Acid (2-PMPA) as Orally Available Glutamate Carboxypeptidase II Inhibitors.ACS Med Chem Lett. 2025 Aug 19:10.1021/acsmedchemlett.5c00384. doi: 10.1021/acsmedchemlett.5c00384. Online ahead of print. ACS Med Chem Lett. 2025. PMID: 40881187 Free PMC article.
-
Calcium waste as a catalyst in the transesterification for demanding esters: scalability perspective.Beilstein J Org Chem. 2025 Jul 28;21:1520-1527. doi: 10.3762/bjoc.21.114. eCollection 2025. Beilstein J Org Chem. 2025. PMID: 40761764 Free PMC article.
References
-
- Yanni S. and Thakker D. R., in Prodrugs: Challenges and Rewards Part 1, ed. V. J. Stella, R. T. Borchardt, M. J. Hageman, R. Oliyai, H. Maag and J. W. Tilley, Springer New York, 2007, pp. 1043–1081
Publication types
LinkOut - more resources
Full Text Sources

