Design and synthesis of cyclic lipidated peptides derived from the C-terminus of Cx43 for hemichannel inhibition and cardiac endothelium targeting
- PMID: 39829973
- PMCID: PMC11740094
- DOI: 10.1039/d4md00850b
Design and synthesis of cyclic lipidated peptides derived from the C-terminus of Cx43 for hemichannel inhibition and cardiac endothelium targeting
Abstract
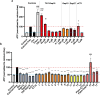
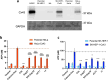
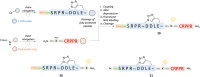
A peptide segment that is 10 residues long at the C-terminal (CT) region of Cx43 is known to be involved in interactions, both with the Cx43 protein itself and with other proteins, that result in hemichannel (HC) activity regulation. Previously reported mimetic peptides based on this region (e.g., αCT1, CT10) have been revealed to be promising therapeutic agents in the context of cardiovascular diseases. In this work, novel approaches, such as C- and N-terminal modification and cyclization, to improve the proteolytic stability and bioavailability of the CT10 peptide are presented. These efforts resulted in a set of unprecedented potent cyclic inhibitors of HC-mediated ATP release with a half-life largely exceeding 24 hours. Additionally, the introduction of a lipophilic moiety with different solubilizing linkers led to the generation of a novel series of water-soluble and lipidated peptides that exhibited high inhibitory capacity in in vitro assays at submicromolar concentrations. A cardiac endothelium targeting strategy was also adopted, exploiting the ability of the CRPPR peptide to selectively deliver the peptides to endothelial cells.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures







References
-
- Kwak B. R. and Nielsen M. S., in Zipes and Jalife's Cardiac Electrophysiology: From Cell to Bedside, ed. J. Jalife and W. G. Stevenson, Elsevier, Philadelphia, PA, USA, 8th edn, 2021, ch. 15, pp. 168–177
LinkOut - more resources
Full Text Sources

