Friends and foes: symbiotic and algicidal bacterial influence on Karenia brevis blooms
- PMID: 39830096
- PMCID: PMC11740886
- DOI: 10.1093/ismeco/ycae164
Friends and foes: symbiotic and algicidal bacterial influence on Karenia brevis blooms
Abstract
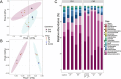
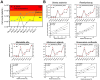
Harmful Algal Blooms (HABs) of the toxigenic dinoflagellate Karenia brevis (KB) are pivotal in structuring the ecosystem of the Gulf of Mexico (GoM), decimating coastal ecology, local economies, and human health. Bacterial communities associated with toxigenic phytoplankton species play an important role in influencing toxin production in the laboratory, supplying essential factors to phytoplankton and even killing blooming species. However, our knowledge of the prevalence of these mechanisms during HAB events is limited, especially for KB blooms. Here, we introduced native microbial communities from the GoM, collected during two phases of a Karenia bloom, into KB laboratory cultures. Using bacterial isolation, physiological experiments, and shotgun metagenomic sequencing, we identified both putative enhancers and mitigators of KB blooms. Metagenome-assembled genomes from the Roseobacter clade showed strong correlations with KB populations during HABs, akin to symbionts. A bacterial isolate from this group of metagenome-assembled genomes, Mameliella alba, alleviated vitamin limitations of KB by providing it with vitamins B1, B7 and B12. Conversely, bacterial isolates belonging to Bacteroidetes and Gammaproteobacteria, Croceibacter atlanticus, and Pseudoalteromonas spongiae, respectively, exhibited strong algicidal properties against KB. We identified a serine protease homolog in P. spongiae that putatively drives the algicidal activity in this isolate. While the algicidal mechanism in C. atlanticus is unknown, we demonstrated the efficiency of C. atlanticus to mitigate KB growth in blooms from the GoM. Our results highlight the importance of specific bacteria in influencing the dynamics of HABs and suggest strategies for future HAB management.
Keywords: HABs; algicidal bacteria; dinoflagellates; harmful algal blooms; phytoplankton–bacteria interactions; vitamins.
© The Author(s) 2024. Published by Oxford University Press on behalf of the International Society for Microbial Ecology.
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Diverse ssRNA viruses associated with Karenia brevis harmful algal blooms in southwest Florida.mSphere. 2025 Apr 29;10(4):e0109024. doi: 10.1128/msphere.01090-24. Epub 2025 Mar 20. mSphere. 2025. PMID: 40111022 Free PMC article.
-
Advection of Karenia brevis blooms from the Florida Panhandle towards Mississippi coastal waters.Harmful Algae. 2018 Feb;72:46-64. doi: 10.1016/j.hal.2017.12.008. Epub 2018 Jan 2. Harmful Algae. 2018. PMID: 29413384
-
Isolation and characterization of an algicidal bacterium against the bloom-forming algae raphidophyte Heterosigma akashiwo.Environ Technol. 2023 Jul;44(17):2607-2616. doi: 10.1080/09593330.2022.2036250. Epub 2022 Feb 19. Environ Technol. 2023. PMID: 35099361
-
Algicidal bacteria in the sea and their impact on algal blooms.J Eukaryot Microbiol. 2004 Mar-Apr;51(2):139-44. doi: 10.1111/j.1550-7408.2004.tb00538.x. J Eukaryot Microbiol. 2004. PMID: 15134248 Review.
-
Marine harmful algal blooms (HABs) in the United States: History, current status and future trends.Harmful Algae. 2021 Feb;102:101975. doi: 10.1016/j.hal.2021.101975. Epub 2021 Mar 3. Harmful Algae. 2021. PMID: 33875183 Free PMC article. Review.
Cited by
-
Structural and regulatory determinants of flagellar motility in Rhodobacterales - The archetypal flagellum of Phaeobacter inhibens DSM 17395.bioRxiv [Preprint]. 2025 Mar 24:2025.03.24.645028. doi: 10.1101/2025.03.24.645028. bioRxiv. 2025. Update in: mSystems. 2025 Aug 19;10(8):e0041925. doi: 10.1128/msystems.00419-25. PMID: 40196601 Free PMC article. Updated. Preprint.
-
Structural and regulatory determinants of flagellar motility in Rhodobacterales-the archetypal flagellum of Phaeobacter inhibens DSM 17395.mSystems. 2025 Aug 19;10(8):e0041925. doi: 10.1128/msystems.00419-25. Epub 2025 Jul 8. mSystems. 2025. PMID: 40626787 Free PMC article.
References
-
- Landsberg JH. The effects of harmful algal blooms on aquatic organisms. Rev Fish Sci 2002;10:113–390. 10.1080/20026491051695 - DOI
-
- Anderson DM, Glibert PM, Burkholder JM. Harmful algal blooms and eutrophication: nutrient sources, composition, and consequences. Estuaries 2002;25:704–26. 10.1007/BF02804901 - DOI
-
- Franks PJ. Recent advances in modelling of harmful algal blooms. In: Glibert PM, Berdalet E, Burford MA, Pitcher GC, Zhou M (eds), Global Ecology and Oceanography of Harmful Algal Blooms .Cham: Springer, 2018, 359–77. 10.1007/978-3-319-70069-4_19 - DOI
-
- Glibert PM, Allen JI, Bouwman Aet al. . Modeling of HABs and eutrophication: status, advances, challenges. J Mar Syst 2010;83:262–75. 10.1016/j.jmarsys.2010.05.004 - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

