Human stem cell models for Marfan syndrome: a brief overview of the rising star in disease modelling
- PMID: 39830211
- PMCID: PMC11739147
- DOI: 10.3389/fcell.2024.1498669
Human stem cell models for Marfan syndrome: a brief overview of the rising star in disease modelling
Abstract
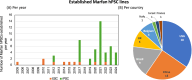
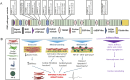
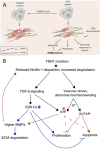
The introduction of pluripotent stem cells into the field of disease modelling resulted in numerous opportunities to study and uncover disease mechanisms in a petri dish. This promising avenue has also been applied to model Marfan syndrome, a disease affecting multiple organ systems, including the skeletal and cardiovascular system. Marfan syndrome is caused by pathogenic variants in FBN1, the gene encoding for the extracellular matrix protein fibrillin-1 which ensembles into microfibrils. There is a poor genotype-phenotype correlation displayed by the diverse clinical manifestations of this disease in patients. Up to now, 52 different human pluripotent stem cells lines have been established and reported for Marfan syndrome. These stem cells have been employed to model aortopathy, skeletal abnormalities and cardiomyopathy in vitro. These models were able to recapitulate key features of the disease that are also observed in patients. The use of pluripotent stem cells will help to uncover disease mechanisms and to identify new therapeutic strategies in Marfan syndrome.
Keywords: Marfan syndrome; aortopathy; cardiomyopathy; disease modelling; human pluripotent stem cells; in vitro.
Copyright © 2025 Aalders, Muiño Mosquera and van Hengel.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Aalberts J. J., Schuurman A. G., Pals G., Hamel B. J., Bosman G., Hilhorst-Hofstee Y., et al. (2010). Recurrent and founder mutations in The Netherlands: extensive clinical variability in Marfan syndrome patients with a single novel recurrent fibrillin-1 missense mutation. Heart J. 18, 85–89. 10.1007/bf03091743 - DOI - PMC - PubMed
-
- Aalders J., Léger L., Demolder A., Muiño Mosquera L., Coucke P., Menten B., et al. (2023). Generation of human induced pluripotent stem cell line UGENTi001-A from a patient with Marfan syndrome carrying a heterozygous c.7754T > C variant in FBN1 and the isogenic control UGENT001-A-1 using CRISPR/Cas9 editing. Stem Cell Res. 67, 103036. 10.1016/j.scr.2023.103036 - DOI - PubMed
-
- Aalders J., Léger L., Van der Meeren L., Sinha S., Skirtach A. G., De Backer J., et al. (2024). Three-dimensional co-culturing of stem cell-derived cardiomyocytes and cardiac fibroblasts reveals a role for both cell types in Marfan-related cardiomyopathy. Matrix Biol. 126, 14–24. 10.1016/j.matbio.2024.01.003 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

