A phylogenetic assessment of Akanthomyces sensu lato in Cordycipitaceae (Hypocreales, Sordariomycetes): introduction of new genera, and the resurrection of Lecanicillium
- PMID: 39830296
- PMCID: PMC11736260
- DOI: 10.3114/fuse.2024.14.17
A phylogenetic assessment of Akanthomyces sensu lato in Cordycipitaceae (Hypocreales, Sordariomycetes): introduction of new genera, and the resurrection of Lecanicillium
Abstract
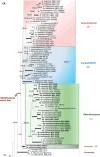
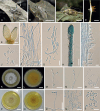
During entomopathogenic fungal surveys conducted in Thailand, 15 specimens tentatively classified under Akanthomyces sensu lato were identified. To gain a comprehensive understanding of their taxonomy, molecular phylogenies using combined LSU, TEF1, RPB1, and RPB2 sequence data, together with morphological examination of several Akanthomyces spp. from previous studies were conducted. The analyses revealed distinct clades representing independent lineages within the Cordycipitaceae. These clades were further characterized by different asexual morph types and the respective hosts they parasitize. In this context, we resurrected the genus Lecanicillium to accommodate 12 known species previously classified under Akanthomyces sensu lato, found on diverse hosts. We propose four new genera - Corniculantispora, Corpulentispora, Zarea, and Zouia - from species previously identified as Lecanicillium. Notably, certain Akanthomyces species associated with spiders and parasitic on Ophiocordyceps sinensis were reclassified into the new genera Arachnidicola and Kanoksria, respectively. Moreover, we introduce four novel species in Akanthomyces sensu stricto found across a diverse range of moth families: Ak. buriramensis, Ak. fusiformis, Ak. niveus, and Ak. phariformis. Additionally, we provide descriptions and illustrations of the sexual morph linked to Ak. laosensis and Ak. pseudonoctuidarum, along with a second type of synnemata seen in Ak. noctuidarum and Ak. pseudonoctuidarum. To assist with their identification, keys to the genera Akanthomyces, Arachnidicola, and Lecanicillium are provided, but should not be used to replace molecular identification. Citation: Khonsanit A, Thanakitpipattana D, Mongkolsamrit S, Kobmoo N, Phosrithong N, Samson RA, Crous PW, Luangsa-ard JJ (2024). A phylogenetic assessment of Akanthomyces sensu lato in Cordycipitaceae (Hypocreales, Sordariomycetes): introduction of new genera, and the resurrection of Lecanicillium. Fungal Systematics and Evolution 14: 271-305. doi: 10.3114/fuse.2024.14.17.
Keywords: Cordycipitaceae; molecular phylogeny; new taxa; taxonomy.
© 2024 Westerdijk Fungal Biodiversity Institute.
Figures








Similar articles
-
Molecular Phylogenetics and Estimation of Evolutionary Divergence and Biogeography of the Family Cordycipitaceae (Ascomycota, Hypocreales).J Fungi (Basel). 2025 Jan 2;11(1):28. doi: 10.3390/jof11010028. J Fungi (Basel). 2025. PMID: 39852447 Free PMC article.
-
Revisiting Metarhizium and the description of new species from Thailand.Stud Mycol. 2020 May 5;95:171-251. doi: 10.1016/j.simyco.2020.04.001. eCollection 2020 Mar. Stud Mycol. 2020. PMID: 32855740 Free PMC article.
-
Species diversity and major host/substrate associations of the genus Akanthomyces (Hypocreales, Cordycipitaceae).MycoKeys. 2024 Jan 15;101:113-141. doi: 10.3897/mycokeys.101.109751. eCollection 2024. MycoKeys. 2024. PMID: 38269036 Free PMC article.
-
New mycoparasitic species in the genera Niveomyces and Pseudoniveomycesgen. nov. (Hypocreales: Cordycipitaceae), with sporothrix-like asexual morphs, from Thailand.Fungal Syst Evol. 2023 Nov;12:91-110. doi: 10.3114/fuse.2023.12.07. Epub 2023 Aug 28. Fungal Syst Evol. 2023. PMID: 38533477 Free PMC article.
-
Disentangling cryptic species with isaria-like morphs in Cordycipitaceae.Mycologia. 2018 Jan-Feb;110(1):230-257. doi: 10.1080/00275514.2018.1446651. Mycologia. 2018. PMID: 29863995
Cited by
-
New treasures in Cordycipitaceae: Fungicolous fungi associated with Pseudocercospora fijiensis and P. musae in Brazil, including Matutinistella gen. nov.Fungal Syst Evol. 2025 Jun;15:133-152. doi: 10.3114/fuse.2025.15.06. Epub 2024 Oct 18. Fungal Syst Evol. 2025. PMID: 40170761 Free PMC article.
-
Molecular Phylogenetics and Estimation of Evolutionary Divergence and Biogeography of the Family Cordycipitaceae (Ascomycota, Hypocreales).J Fungi (Basel). 2025 Jan 2;11(1):28. doi: 10.3390/jof11010028. J Fungi (Basel). 2025. PMID: 39852447 Free PMC article.
-
Four new araneogenous species and a new genus in Hypocreales (Clavicipitaceae, Cordycipitaceae) from the karst region of China.MycoKeys. 2025 Jan 23;112:335-359. doi: 10.3897/mycokeys.112.140799. eCollection 2025. MycoKeys. 2025. PMID: 39897122 Free PMC article.
-
Molecular phylogeny and morphology reveal four novel species in Cordycipitaceae in China.MycoKeys. 2025 Apr 9;116:91-124. doi: 10.3897/mycokeys.116.147006. eCollection 2025. MycoKeys. 2025. PMID: 40248652 Free PMC article.
References
-
- Arnold G, Castañeda Ruiz RF. (1987) Neue HyphomyzetenArten aus Kuba II. Verticillium antillanum, Nakatea curvularioides und Cladobotryum cubitensis. Feddes Repertorium Specierum Novarum Regni Vegetabilis 98: 411–417.
LinkOut - more resources
Full Text Sources
Miscellaneous
