Protective effects and possible mechanisms of mesenchymal stem cells and mesenchymal stem cell-derived extracellular vesicles against kidney fibrosis in animal models: a systematic review and meta-analysis
- PMID: 39830341
- PMCID: PMC11739157
- DOI: 10.3389/fphar.2024.1511525
Protective effects and possible mechanisms of mesenchymal stem cells and mesenchymal stem cell-derived extracellular vesicles against kidney fibrosis in animal models: a systematic review and meta-analysis
Abstract
Introduction: The risk of kidney fibrosis is significantly elevated in individuals with diabetes, chronic nephritis, trauma, and other underlying conditions. Concurrently, human umbilical cord blood-derived mesenchymal stem cells (hUCB-MSCs) and their extracellular vesicles (MSC-Exos) have gained prominence in regenerative medicine. In light of these observations, we are undertaking a meta-analysis to elucidate the influence of hUCB-MSCs and MSC-Exos on kidney fibrosis.
Methods: To identify eligible trials, we conducted a comprehensive search of the CNKI, PubMed, Web of Science and Wanfang databases from inception to 24 October 2022. Furthermore, the methodological quality of the included studies was evaluated using the Systematic Review Center for Laboratory Animal Experimentation (SYRCLE) risk-of-bias tool. Besides, the weighted standard mean difference (SMD) with a 95% confidence interval (CI) was calculated using the Review Manager 5.4 software. The Stata (12.0) software was employed to assess the impact of factors on outcome heterogeneity and publication bias in the study. A total of 645 related research studies were retrieved, of which 14 that involved 219 experimental animals were included in the study.
Results: In comparison to the control treatment, treatment with Human UCB MSC and MSC-Exos was observed to significantly enhance renal function in animal models of kidney fibrosis. This was evidenced by a reduction in serum creatinine (Scr) levels (p < 0.00001) and blood urea nitrogen (BUN) levels (p < 0.00001), as well as reduction of CD68+ macrophages (p < 0.00001), TdT-mediated dUTP Nick-End labeling (TUNEL)+ tubular cells(p < 0.00001), α-SMA levels (p = 0.0009) and TGF-β1 (p < 0.00001). P < 0.05 is deemed to indicate a statistically significant difference. Alpha-smooth muscle actin (α-SMA) is a specific protein that is normally expressed in myofibroblasts. The term "CD68+ macrophages" refers to macrophages that express the CD68 protein on their cell surface. Both macrophages and myofibroblasts have been linked to the development of kidney fibrosis. In this study, the quantity of CD68+ macrophages and α-SMA was employed as a means of gauging the extent of renal fibrosis. Additionally, transforming growth factor beta 1 (TGF-β1) is a significant cytokine implicated in the pathogenesis of kidney fibrosis. TUNEL-positive tubular cells represent tubular cells undergoing apoptosis. It is hypothesized that this may result in a reduction of tubular apoptosis and a delay in kidney fibrosis, due to the inhibition of the transformation of macrophages into myofibroblasts (MMT) and the disruption of the kidney fibrogenic niche.
Conclusion: The principal findings of this preclinical systematic review indicate that hUCB MSC and MSC-Exos have a substantial protective impact against kidney fibrosis. Kidney transfer remains the final option for traditional renal fibrosis treatment. The lack of donors and high cost make it challenging for many patients to access appropriate treatment. Although this study still suffers from three shortcomings: sample size, methodological consistency and translational challenges, the hUCB MSC and MSC-Exos have been demonstrated to reduce tubular apoptosis and inhibit fibrotic progression. The hUCB MSC and MSC-Exos offer a promising alternative due to their lower price and accessibility. Nevertheless, further high-quality studies are required in the future to address the methodological limitations identified in this review.
Systematic review registration: Identifier INPLASY2022100104.
Keywords: extracellular vesicle; kidney fibrosis; mesenchymal stem cell; mice; systemic review.
Copyright © 2025 Lv, Hua and Lu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




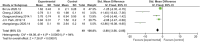

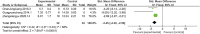
Similar articles
-
Extracellular vesicles for ischemia/reperfusion injury-induced acute kidney injury: a systematic review and meta-analysis of data from animal models.Syst Rev. 2022 Sep 8;11(1):197. doi: 10.1186/s13643-022-02003-5. Syst Rev. 2022. PMID: 36076305 Free PMC article.
-
Administration of mesenchymal stem cells in diabetic kidney disease: a systematic review and meta-analysis.Stem Cell Res Ther. 2021 Jan 7;12(1):43. doi: 10.1186/s13287-020-02108-5. Stem Cell Res Ther. 2021. PMID: 33413678 Free PMC article.
-
Micro-vesicles from mesenchymal stem cells over-expressing miR-34a inhibit transforming growth factor-β1-induced epithelial-mesenchymal transition in renal tubular epithelial cells in vitro.Chin Med J (Engl). 2020 Apr 5;133(7):800-807. doi: 10.1097/CM9.0000000000000720. Chin Med J (Engl). 2020. PMID: 32149762 Free PMC article.
-
Meta-analysis study of the therapeutic impact of Mesenchymal stem cells derived exosomes for chronic kidney diseases.Biochem Biophys Rep. 2025 Jun 3;43:102072. doi: 10.1016/j.bbrep.2025.102072. eCollection 2025 Sep. Biochem Biophys Rep. 2025. PMID: 40524833 Free PMC article.
-
Exosomes From Human Umbilical Cord Stem Cells Suppress Macrophage-to-myofibroblast Transition, Alleviating Renal Fibrosis.Inflammation. 2024 Dec;47(6):2094-2107. doi: 10.1007/s10753-024-02027-0. Epub 2024 Apr 25. Inflammation. 2024. PMID: 38662165
Cited by
-
Advancements and emerging trends in photodynamic therapy: innovations in cancer treatment and beyond.Photochem Photobiol Sci. 2025 Jul 24. doi: 10.1007/s43630-025-00765-0. Online ahead of print. Photochem Photobiol Sci. 2025. PMID: 40705276 Review.
References
-
- Deng K., Lin D. L., Hanzlicek B., Balog B., Penn M. S., Kiedrowski M. J., et al. (2015). Mesenchymal stem cells and their secretome partially restore nerve and urethral function in a dual muscle and nerve injury stress urinary incontinence model. Am. J. Physiol. Ren. Physiol. 308 (2), F92–F100. 10.1152/ajprenal.00510.2014 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

