Bioequivalence study of fluticasone propionate nebuliser suspensions in healthy Chinese subjects
- PMID: 39830354
- PMCID: PMC11738769
- DOI: 10.3389/fphar.2024.1452596
Bioequivalence study of fluticasone propionate nebuliser suspensions in healthy Chinese subjects
Abstract
Background: Fluticasone propionate is a synthetic trifluoro-substituted glucocorticoid, a highly selective glucocorticoid receptor agonist. Fluticasone propionate nebuliser suspensions is an inhaled corticosteroid with the low systemic bioavailability which provides a low risk (benefit outcome without the adverse effects that accompany systemically administered corticosteroids), referred as a first-line preventive agent for patients with persistent asthma. China has become one of the countries with the highest asthma mortality rate in the world in the past years. It urgently needs good generic drugs to help ease patients' burden and improve their quality of life.
Objective: The primary objective of this study was to evaluate the bioequivalence of fluticasone propionate nebuliser suspensions between test formulation (generic product) and reference formulation (original product, Flixotide Nebules®) with the pharmacokinetic parameters as the endpoint indicators and the secondary objective was to evaluate the safety of two inhalated fluticasone propionate nebuliser suspensions under the condition of fasting in healthy Chinese subjects.
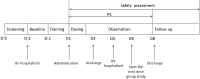
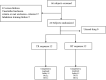
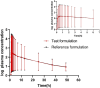
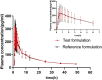
Methods: The bioequivalence study was conducted with a single-center, randomized, open-label, single-dose, two sequences, two-period crossover design. 24 healthy subjects were randomly assigned into T-R and R-T sequence groups with 12 patients in each group. The subjects were administered 1 mg (2 mL:0.5 mg,plastic ampoules) of generic fluticasone propionate nebuliser suspension as a test formulation or Flixotide Nebules® as reference formulation and cross administration after sufficient washout period (5 days) for the second period study. The blood sample was collected at predetermined time points up to 48 h and the plasma concentration of fluticasone propionate was determined by HPLC-MS/MS in healthy subjects after inhalation of test or reference formulation. The non-compartment model method (NCA module) of the WinNonlin® software (version 8.3) was used to calculate the pharmacokinetic parameters (Cmax, AUC0-t, AUC0-∞) between the test formulation and the reference formulation were within the predefined range of 80.00% and 125.00%, bioequivalence of both formulations was demonstrated.
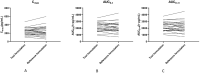
Results: The 90% confidence intervals of the T/R ratio of the geometric mean of Cmax, AUC0-t, and AUC0-∞ for both formulations were 90.24%-112.68%, 96.99%-112.27% and 96.41%-111.59% respectively, which were all within the bioequivalent range of 80%-125%. No severe, suspicious or unexpected serious adverse reactions were reported.
Conclusion: The test and reference formulations of fluticasone propionate nebuliser suspension were pharmacokinetic bioequivalent and were well tolerated and safe in all subjects.
Keywords: asthma; bioequivalence; fluticasone propionate; healthy Chinese subjects; safety.
Copyright © 2025 Cheng, Shen, Zhang, Lei, Zhu, Luo and Xiao.
Conflict of interest statement
Authors TS and GL were employed by Shanghai Chenpon Pharmaceutical Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Bioequivalence Study of 2 Formulations of Fluticasone Nasal Spray in Healthy Chinese Volunteers.Clin Pharmacol Drug Dev. 2025 Mar;14(3):270-275. doi: 10.1002/cpdd.1505. Epub 2025 Jan 9. Clin Pharmacol Drug Dev. 2025. PMID: 39789740 Clinical Trial.
-
Bioequivalence Study of Palbociclib Capsules in Healthy Chinese Subjects Under Fasting and Fed Conditions.Clin Drug Investig. 2022 Jan;42(1):53-63. doi: 10.1007/s40261-021-01103-9. Epub 2021 Nov 26. Clin Drug Investig. 2022. PMID: 34837169 Clinical Trial.
-
Bioequivalence study of domperidone dry suspension in healthy Chinese subjects under fasted and fed conditions: An open-label, randomized, single-dose, crossover trial.Int J Clin Pharmacol Ther. 2023 Jul;61(7):320-328. doi: 10.5414/CP204309. Int J Clin Pharmacol Ther. 2023. PMID: 36999513 Clinical Trial.
-
Pharmacokinetics and Bioequivalence of Two Formulations of Azithromycin Tablets: A Randomized, Single-Dose, Three-Period, Crossover Study in Healthy Chinese Volunteers Under Fasting and Fed Conditions.Drugs R D. 2024 Jun;24(2):201-209. doi: 10.1007/s40268-024-00464-8. Epub 2024 May 30. Drugs R D. 2024. PMID: 38811485 Free PMC article. Clinical Trial.
-
Pharmacokinetics and Bioequivalence of Apremilast Tablets in Chinese Healthy Subjects Under Fasting and Postprandial States.Drug Des Devel Ther. 2024 Jun 14;18:2273-2285. doi: 10.2147/DDDT.S461771. eCollection 2024. Drug Des Devel Ther. 2024. PMID: 38895175 Free PMC article. Clinical Trial.
References
-
- Al-Moamary M. S., Alhaider S. A., Al-Hajjaj M. S., Al-Ghobain M. O., Idrees M. M., Zeitouni M. O., et al. (2012). The Saudi initiative for asthma–2012 update: guidelines for the diagnosis and management of asthma in adults and children. Ann. Thorac. Med. 7 (4), 175–204. 10.4103/1817-1737.102166 - DOI - PMC - PubMed
-
- Al-Numani D., Colucci P., Ducharme M. P. (2015). Rethinking bioequivalence and equivalence requirements of orally inhaled drug products. Asian J. Pharm. Sci. 10 (6), 461–471. 10.1016/j.ajps.2015.08.006 - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

