A tactfully designed photothermal agent collaborating with ascorbic acid for boosting maxillofacial wound healing
- PMID: 39830404
- PMCID: PMC11737384
- DOI: 10.1093/nsr/nwae426
A tactfully designed photothermal agent collaborating with ascorbic acid for boosting maxillofacial wound healing
Abstract
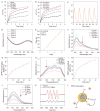
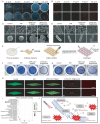
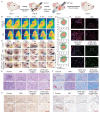
Maxillofacial injuries that may cause severe functional and aesthetic damage require effective and immediate management due to continuous exposure to diverse microbial populations. Moreover, drug resistance, biofilm formation, and oxidative stress significantly impede timely bacterial removal and immune function, making the exploration of advanced materials for maxillofacial wound healing an appealing yet highly challenging task. Herein, a near-infrared photothermal sterilization agent was designed, encapsulated with liposomes and coated with ascorbic acid known for its antioxidant and immune-regulatory functions. The resulting nanoparticles, 4TPE-C6T-TD@AA, effectively neutralize reactive oxygen species generated by lipopolysaccharides, facilitate the conversion of pro-inflammatory M1 macrophages to anti-inflammatory M2 macrophages, and eliminate >90% of Staphylococcus aureus and Escherichia coli by disrupting bacterial physiological functions upon exposure to 808 nm laser irradiation. In vivo experiments demonstrate that 4TPE-C6T-TD@AA rapidly eliminates bacteria from infected wounds in the maxillofacial region of rats, and significantly promotes healing in S. aureus-infected wounds by enhancing collagen formation and modulating the inflammatory microenvironment. In conclusion, this study presents a promising therapeutic strategy for effectively combating bacterial infections and excessive inflammation in treating maxillofacial injuries.
Keywords: anti-inflammation; maxillofacial wound healing; photothermal sterilization.
© The Author(s) 2024. Published by Oxford University Press on behalf of China Science Publishing & Media Ltd.
Figures



Similar articles
-
An injectable multifunctional hydrogel for eradication of bacterial biofilms and wound healing.Acta Biomater. 2023 Apr 15;161:112-133. doi: 10.1016/j.actbio.2023.03.008. Epub 2023 Mar 11. Acta Biomater. 2023. PMID: 36907234
-
Photothermal-responsive nanocomposite injectable hydrogel capable of programed peroxidase-mimicking catalysis and immunomodulation and revascularization enables efficient drug-resistant bacterial elimination and comprehensive tissue regeneration.J Colloid Interface Sci. 2025 Apr;683(Pt 1):752-772. doi: 10.1016/j.jcis.2024.12.120. Epub 2024 Dec 16. J Colloid Interface Sci. 2025. PMID: 39708727
-
Chitosan-based hydrogel incorporated with polydopamine and protoporphyrin for photothermal-oxidation sterilization of bacteria-infected wound therapy.J Colloid Interface Sci. 2025 Jan 15;678(Pt C):89-100. doi: 10.1016/j.jcis.2024.09.098. Epub 2024 Sep 13. J Colloid Interface Sci. 2025. PMID: 39277956
-
Self-Healing Conductive Hydrogels with Dynamic Dual Network Structure Accelerate Infected Wound Healing via Photothermal Antimicrobial and Regulating Inflammatory Response.ACS Appl Mater Interfaces. 2024 Jun 19;16(24):30776-30792. doi: 10.1021/acsami.4c04113. Epub 2024 Jun 7. ACS Appl Mater Interfaces. 2024. PMID: 38848491
-
Controlled-temperature photothermal and oxidative bacteria killing and acceleration of wound healing by polydopamine-assisted Au-hydroxyapatite nanorods.Acta Biomater. 2018 Sep 1;77:352-364. doi: 10.1016/j.actbio.2018.07.030. Epub 2018 Jul 17. Acta Biomater. 2018. PMID: 30030176
Cited by
-
Piezoelectric dual-network tough hydrogel with on-demand thermal contraction and sonopiezoelectric effect for promoting infected-joint-skin-wound healing via FAK and AKT signaling pathways.Natl Sci Rev. 2025 Mar 29;12(5):nwaf118. doi: 10.1093/nsr/nwaf118. eCollection 2025 May. Natl Sci Rev. 2025. PMID: 40309345 Free PMC article.
References
LinkOut - more resources
Full Text Sources
