Asthma Biologics Across the T2 Spectrum of Inflammation in Severe Asthma: Biomarkers and Mechanism of Action
- PMID: 39830595
- PMCID: PMC11742565
- DOI: 10.2147/JAA.S496630
Asthma Biologics Across the T2 Spectrum of Inflammation in Severe Asthma: Biomarkers and Mechanism of Action
Abstract
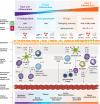
Airway inflammation, a hallmark feature of asthma, drives many canonical features of the disease, including airflow limitation, mucus plugging, airway remodeling, and hyperresponsiveness. The T2 inflammatory paradigm is firmly established as the dominant mechanism of asthma pathogenesis, largely due to the success of inhaled corticosteroids and biologic therapies targeting components of the T2 pathway, including IL-4, IL-5, IL-13, and thymic stromal lymphopoietin (TSLP). However, up to 30% of patients may lack signatures of meaningful T2 inflammation (ie, T2 low). In T2-low asthma patients, T2 inflammation may be masked due to anti-inflammatory treatments or may be highly variable depending on exposure to common asthma triggers such as allergens, respiratory infections, and smoke or pollution. The epithelium and epithelial cytokines (TSLP, IL-33) are increasingly recognized as upstream drivers of canonical T2 pathways and as modulators of various effector cells, including mast cells, eosinophils, and neutrophils, which impact the pathological manifestations of airway smooth muscle hypertrophy, hypercontractility, and airway hyperresponsiveness. Approved biologics for severe asthma target several distinct mechanisms of action, leading to differential effects on the spectrum of T2 inflammation, inflammatory biomarkers, and treatment efficacy (reducing asthma exacerbations, improving lung function, and diminishing symptoms). The approved anti-asthma biologics primarily target T2 immune pathways, with little evidence suggesting a benefit of targeting non-T2 asthma-associated mediators. Indeed, many negative results challenge current assumptions about the etiology of non-T2 asthma and raise doubts about the viability of targeting popular alternative inflammatory pathways, such as T17. Novel data have emerged from the use of biologics to treat various inflammatory mediators and have furthered our understanding of pathogenic mechanisms that drive asthma. This review discusses inflammatory pathways that contribute to asthma, quantitatively outlines effects of available biologics on biomarkers, and summarizes data and challenges from clinical trials that address non-T2 mechanisms of asthma.
Keywords: T2 inflammation; TSLP; anti-TSLP; asthma; biologics; biomarker; thymic stromal lymphopoietin.
© 2025 Lindsley et al.
Conflict of interest statement
A Lindsley and JR Parnes are employees and stockholders of Amgen Inc. J Spahn and KAG Reeh are employees and stockholders of AstraZeneca. N Lugogo received consulting fees from Amgen, AstraZeneca, Avillion, Genentech, GSK, Novartis, Regeneron, Sanofi, and Teva; honoraria for nonspeaker bureau presentations from GSK, Niox, and AstraZeneca; and travel support from AstraZeneca, Sanofi, and GSK; her institution received research support from Amgen, AstraZeneca, Avillion, Evidera, Gossamer Bio, Genentech, GSK, Janssen, Regeneron, Sanofi, Novartis, and Teva. She is an honorary faculty member of Observational and Pragmatic Research Institute, but does not receive compensation for this role.
Figures
References
-
- The global asthma report 2018. Global Asthma Network. Available from: http://globalasthmareport.org/2018/resources/Global_Asthma_Report_2018.pdf. Accessed July 24, 2024.
-
- GINA. GINA report, global strategy for asthma management and prevention 2023. Global Initiative for Asthma; 2023. Available from: https://ginasthma.org/2023-gina-main-report/. Accessed December 13, 2024.
-
- FasenraTM (benralizumab) [prescribing information]. AstraZeneca Pharmaceuticals, LP. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761070s000lbl.pdf. Accessed March 2024.
-
- NucalaTM (mepolizumab) [prescribing information]. GlaxoSmithKline LLC. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/761122s008,125.... Accessed March 2024.
Publication types
LinkOut - more resources
Full Text Sources

