Current status of the development of dengue vaccines
- PMID: 39830640
- PMCID: PMC11741033
- DOI: 10.1016/j.jvacx.2024.100604
Current status of the development of dengue vaccines
Abstract
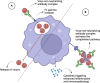
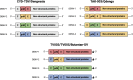
Dengue fever is caused by the mosquito-borne dengue virus (DENV), which is endemic in more than 100 countries. Annually, there are approximately 390 million dengue cases, with a small subset manifesting into severe illnesses, such as dengue haemorrhagic fever or dengue shock syndrome. Current treatment options for dengue infections remain supportive management due to the lack of an effective vaccine and clinically approved antiviral. Although the CYD-TDV (Dengvaxia®) vaccine with an overall vaccine efficacy of 60 % has been licensed for clinical use since 2015, it poses an elevated risk of severe dengue infections especially in dengue-naïve children below 9 years of age. The newly approved Qdenga vaccine was able to achieve an overall vaccine efficacy of 80 % after 12 months, but it was not able to provide a protective effect against DENV-3 in dengue naïve individuals. The Butantan-DV vaccine candidate is still undergoing phase 3 clinical trials for safety and efficacy evaluations in humans. Apart from live-attenuated vaccines, various other vaccine types are also currently being studied in preclinical and clinical studies. This review discusses the current status of dengue vaccine development.
Keywords: Dengue virus; Flavivirus; Live-attenuated vaccine; Vaccine; Vaccine development.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- Ahmed A.M., et al. Prevalence and burden of dengue infection in Europe: a systematic review and meta-analysis. Rev Med Virol. 2020;30(2) - PubMed
-
- Zhang J., et al. Co-circulation of three dengue virus serotypes led to a severe dengue outbreak in Xishuangbanna, a border area of China, Myanmar, and Laos, in 2019. Int J Infect Dis. 2021;107:15–17. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

