Successful long-term outcome of neoadjuvant sequential targeted therapy and chemotherapy for stage III non-small cell lung carcinoma: 10 case series
- PMID: 39830752
- PMCID: PMC11736588
- DOI: 10.21037/tlcr-24-545
Successful long-term outcome of neoadjuvant sequential targeted therapy and chemotherapy for stage III non-small cell lung carcinoma: 10 case series
Abstract
Background: Perioperative treatment of locally advanced non-small cell lung cancer (NSCLC) is attracting attention. The effect of neoadjuvant tyrosine kinase inhibitor (TKI) therapy on postoperative long-term outcomes in patients with driver gene mutations remains unclear. The aim of this study was to clarify the long-term survival outcomes of patients with stage III NSCLC harboring driver gene mutations who received preoperative TKI therapy.
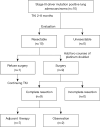
Methods: Between January 2016 and December 2018, 10 patients with clinical stage III NSCLC with driver gene mutations were treated with TKIs [epidermal growth factor receptor (EGFR) mutation, n=9; anaplastic lymphoma kinase (ALK) fusion, n=1]. One patient refused surgery. The remaining nine patients received sequential chemotherapy followed by surgery. Postoperatively, six patients received adjuvant chemotherapy, and TKIs were readministered in four patients.
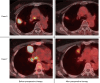
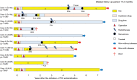
Results: The main adverse events of TKIs were grade 3 liver damage and grade 3 skin rash, which required a change in the drug from gefitinib to afatinib and dose reduction, respectively. In all 10 patients, the radiological response to TKIs was greater than the partial response, and nine patients underwent radical surgery. Although viable cancer cells remained in all patients with EGFR mutations, a pathological complete response was obtained in the patient with ALK fusion. No mortality or major morbidity was observed perioperatively. Of the patients who underwent surgery, 3 were alive without recurrence, while 6 had distant metastasis, including 5 with brain metastasis. Seven of the nine patients who underwent surgery were still alive after a median follow-up period of 77.2 months.
Conclusions: Successful long-term outcomes were achieved after sequential targeted therapy and chemotherapy, followed by surgery for stage III NSCLC. However, it is noteworthy that postoperative treatment may have also contributed to minimizing postoperative recurrence.
Keywords: Non-small cell lung cancer with driver gene mutation (NSCLC with driver gene mutation); case series; long-term survival outcome; neoadjuvant tyrosine kinase inhibitors (neoadjuvant TKIs); stage III.
2024 AME Publishing Company. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-545/coif). The authors have no conflicts of interest to declare.
Figures
References
-
- NCCN Guidelines. Non-Small Cell Lung Cancer Version 5.2024 [Last accessed on 15 May 2024]. Available online: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf
-
- Aoki M, Miyata R, Kamimura G, et al. Effect of Tegafur-Uracil in Resected Stage IB Lung Adenocarcinoma According to Presence or Absence of Epidermal Growth Factor Receptor Gene Mutation: A Retrospective Cohort Study. Ann Thorac Cardiovasc Surg 2024;30. doi: . 10.5761/atcs.oa.23-00134 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
