A retrospective study of the efficacy and safety of immune checkpoint inhibitors combined with chemotherapy for the treatment of SMARCA4-deficient thoracic tumors
- PMID: 39830761
- PMCID: PMC11736595
- DOI: 10.21037/tlcr-24-691
A retrospective study of the efficacy and safety of immune checkpoint inhibitors combined with chemotherapy for the treatment of SMARCA4-deficient thoracic tumors
Abstract
Background: Thoracic tumors characterized by a deficiency in SMARCA4 are highly aggressive and linked to a poor prognosis. This retrospective study explores the efficacy and safety of immune checkpoint inhibitors (ICIs) in combination with chemotherapy for SMARCA4-deficient undifferentiated tumors (SMARCA4-dUT) and SMARCA4-deficient non-small cell lung cancer (SMARCA4-dNSCLC).
Methods: A cohort of 59 individuals was analyzed, including 35 patients with SMARCA4-dUT and 24 with SMARCA4-dNSCLC.
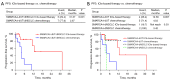
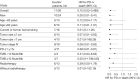
Results: Clinical characteristics as gender, age, smoking status, and metastatic sites did not significantly vary between SMARCA4-dUT and SMARCA4-dNSCLC. Nonsense and frameshift mutations in the SMARCA4 gene can result in the loss of its protein expression. Following a median follow-up of 7.6 months, the median progression-free survival (mPFS) notably increased with ICIs-based combination therapy compared to chemotherapy, the mPFS was 12.60 vs. 4.03 months in the SMARCA4-dUT subgroup (P=0.007) and not reached vs. 3.42 months in the SMARCA4-dNSCLC subgroup (P=0.03). In stage IV patients, the risk of disease progression and death decreased with ICIs-based combination therapy vs. chemotherapy [ICIs-based therapy vs. chemotherapy: hazard ratio (HR) =0.076; 95% confidence interval (CI): 0.009-0.624]. The most prevalent grade 3 or higher adverse events (AEs) in both groups were hematologic decreases, consistent with typical chemotherapy AEs. No treatment-related AEs led to patient fatalities.
Conclusions: The combination of ICIs and chemotherapy is more effective than chemotherapy for patients with advanced SMARCA4-deficient thoracic tumors (SMARCA4-dTT), and the safety is manageable.
Keywords: SMARCA4-deficient non-small cell lung cancer (SMARCA4-dNSCLC); SMARCA4-deficient thoracic tumors (SMARCA4-dTT); SMARCA4-deficient undifferentiated tumors (SMARCA4-dUT); chemotherapy; immune checkpoint inhibitors (ICIs).
2024 AME Publishing Company. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-691/coif). The authors have no conflicts of interest to declare.
Figures



Similar articles
-
[Evaluation of Efficacy and Prognosis Analysis of Stage III-IV SMARCA4-deficient Non-small Cell Lung Cancer Treated by PD-1 Immune Checkpoint Inhibitors plus Chemotherapy and Chemotherapy].Zhongguo Fei Ai Za Zhi. 2023 Sep 20;26(9):659-668. doi: 10.3779/j.issn.1009-3419.2023.101.26. Zhongguo Fei Ai Za Zhi. 2023. PMID: 37985152 Free PMC article. Chinese.
-
Retrospective Insights into the Clinicopathological Features and Treatment Outcomes of Thoracic SMARCA4-Deficient Tumors.Technol Cancer Res Treat. 2025 Jan-Dec;24:15330338251345377. doi: 10.1177/15330338251345377. Epub 2025 May 22. Technol Cancer Res Treat. 2025. PMID: 40400412 Free PMC article.
-
Rare SMARCA4-deficient thoracic tumor: Insights into molecular characterization and optimal therapeutics methods.Lung Cancer. 2024 Jun;192:107818. doi: 10.1016/j.lungcan.2024.107818. Epub 2024 May 9. Lung Cancer. 2024. PMID: 38763102
-
Chemotherapy and Immune Checkpoint Inhibitors in a Case of SMARCA4-dUT: A Case Report and Review of Literature.J Investig Med High Impact Case Rep. 2023 Jan-Dec;11:23247096231176220. doi: 10.1177/23247096231176220. J Investig Med High Impact Case Rep. 2023. PMID: 37269109 Free PMC article. Review.
-
SMARCA4-Deficient Undifferentiated Tumor of Lung Mass-A Rare Tumor With the Rarer Occurrence of Brain Metastasis: A Case Report and Review of the Literature.J Investig Med High Impact Case Rep. 2022 Jan-Dec;10:23247096221074864. doi: 10.1177/23247096221074864. J Investig Med High Impact Case Rep. 2022. PMID: 35356840 Free PMC article. Review.
Cited by
-
The role of SMARCA4 in lung cancer.Sci Rep. 2025 Aug 5;15(1):28605. doi: 10.1038/s41598-025-13913-4. Sci Rep. 2025. PMID: 40764807 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Miscellaneous
