Effectiveness of Distant/Remote Blessing Treatment on Cognitive-motor Function: A Randomized Double-blind Placebo-controlled Trial
- PMID: 39830804
- PMCID: PMC11741897
- DOI: 10.52965/001c.128098
Effectiveness of Distant/Remote Blessing Treatment on Cognitive-motor Function: A Randomized Double-blind Placebo-controlled Trial
Abstract
Background: Biofield therapies can be administered in person (hands-on treatment) or remotely, and this study focuses on the latter. A literature review did not find any reports on the effectiveness of remote biofield energy /blessing therapy in enhancing cognition and motor function performance in adults.
Objective: The aim of this study was to examine the effect of distant/remote blessing (biofield energy) therapy on the cognitive and motor functions in adults with self-reported neuropsychological impairments using NIH Toolbox®.
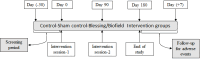
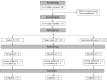
Methods: The present study was a randomized, double-blind clinical trial involving 117 participants with self-reported neuropsychological impairments. These participants were stratified into three distinct groups: control, sham control, and blessing/biofield treatment as the intervention. At baseline (day 0), day 90, and day 180, NIH Toolbox® was employed to evaluate all participants' cognitive and motor function scores.
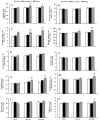
Results: In the blessing treatment group, language function score (p <0.01), working memory (p <0.0001), and episodic memory (p <0.0001) scores exhibited statistically significant differences compared to both the naïve control and sham control groups. Moreover, in the blessing intervention group, a substantial improvement was observed in locomotion (p <0.0001), standing balance (p <0.01), dexterity (p <0.01), grip strength (p <0.05), and muscle endurance (p <0.05) compared to the naïve control and sham control groups. Importantly, no adverse effects were reported during the study period.
Conclusion: The study outcomes revealed that distant/remote blessing/biofield energy therapy is safe, non-invasive, and less expensive. It enhances cognitive-motor functions in adults with perceived neuropsychological impairments.
Clinical trial registration: CTRI/2022/07/043736.
Keywords: Biofield Energy Therapy; Cognitive-motor function; NIH Toolbox®; Neuropsychological impairments; Randomized clinical trial.
Conflict of interest statement
Authors AB, MKT, and DT were employed by Trivedi Global, Inc. Authors SM and SJ were employed by Trivedi Science Research Laboratory Pvt. Ltd.
Figures
Similar articles
-
Assessment of cognitive-motor functions in adults with perceived neuropsychological problems using NIH toolbox after remote biofield energy treatment as non-pharmacological intervention: A randomized double-blind placebo controlled trial.Neuropsychopharmacol Rep. 2024 Dec;44(4):749-761. doi: 10.1002/npr2.12482. Epub 2024 Sep 13. Neuropsychopharmacol Rep. 2024. PMID: 39270308 Free PMC article. Clinical Trial.
-
The Improvement of Cognitive-Motor Function Scores Using the NIH-Toolbox in Adults Subjects After Biofield Energy Therapy: A Randomized Controlled Double-blind Trial.Altern Ther Health Med. 2024 Sep;30(9):28-35. Altern Ther Health Med. 2024. PMID: 39212514 Clinical Trial.
-
Investigation of Non-Pharmacological Distant Energy Therapy in Adults with Self-Perceived Mental and Psychological Health Problems: Proof-of-Concept Randomized Controlled Trial.Altern Ther Health Med. 2025 May;31(3):26-33. Altern Ther Health Med. 2025. PMID: 39899546 Clinical Trial.
-
The use of biofield energy therapy as complementary and alternative medicine in human health care system: a narrative review and potential mechanisms.J Complement Integr Med. 2024 Apr 3;21(4):451-460. doi: 10.1515/jcim-2024-0027. Print 2024 Dec 17. J Complement Integr Med. 2024. PMID: 38563780 Review.
-
Computerised cognitive training for 12 or more weeks for maintaining cognitive function in cognitively healthy people in late life.Cochrane Database Syst Rev. 2020 Feb 27;2(2):CD012277. doi: 10.1002/14651858.CD012277.pub3. Cochrane Database Syst Rev. 2020. PMID: 32104914 Free PMC article.
References
-
- Cognitive rehabilitation interventions for executive function: moving from bench to bedside in patients with traumatic brain injury. Cicerone K., Levin H., Malec J.., et al. 2006J Cogn Neurosci. 18(7):1212–1222. doi: 10.1162/jocn.2006.18.7.1212. https://doi.org/10.1162/jocn.2006.18.7.1212 - DOI - DOI - PubMed
-
- Walking behaviour of healthy elderly: attention should be paid. de Bruin E. D., Schmidt A. 2010Behav Brain Funct. 6:59. doi: 10.1186/1744-9081-6-59. https://doi.org/10.1186/1744-9081-6-59 - DOI - DOI - PMC - PubMed
-
- Measurement of executive function: considerations for detecting adult age differences. Bryan J., Luszcz M. A. 2000J Clin Exp Neuropsychol. 22(1):40–55. doi: 10.1076/1380-3395(200002)22:1;1-8;FT040. https://doi.org/10.1076/1380-3395(200002)22:1;1-8;FT040 - DOI - DOI - PubMed
-
- The role of executive function and attention in gait. Yogev-Seligmann G., Hausdorff J. M., Giladi N. 2008Mov Disord. 23(3):329–342. doi: 10.1002/mds.21720. https://doi.org/10.1002/mds.21720 - DOI - DOI - PMC - PubMed
-
- Evaluate the safety and efficacy of a biofield energy treated proprietary dietary supplement (TRI 360™) on psychological symptoms, mental disorders, emotional well-being, and quality of life in adult subjects. Trivedi M. K., Branton A., Trivedi D.., et al. 2022Altern Ther Health Med. 9:AT7498. doi: 10.3389/fpsyt.2022.919284. - DOI - PubMed
LinkOut - more resources
Full Text Sources

