Preparation and validation of nanomolar aqueous bilirubin standard solutions
- PMID: 39830880
- PMCID: PMC11741073
- DOI: 10.1016/j.mex.2024.103123
Preparation and validation of nanomolar aqueous bilirubin standard solutions
Abstract
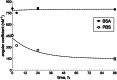
Bilirubin (BR) is the product of cellular heme catabolism and the main bile pigment in animal blood. It is an established biomarker for hemolysis and liver function. Over the last decade, mild hyperbilirubinemia has been shown to be a biomarker for a lower risk of cardiovascular disease, due to its antioxidant and anti-inflammatory effects. In order to use bilirubin as a predictive biomarker, new powerful methods for its direct analysis in human blood are currently being developed. To harmonize the different methods, it is essential to use high-quality BR standard solutions for assay calibration. We present here a protocol for the preparation of stable standard solutions in the range of 10-9- 10-5M BR at pH 7.4 that can facilitate a uniform approach for assay calibration without or with a sample preparation step.•The bilirubin standard solutions are prepared in buffered saline solution at physiological pH (not in alkali, not in apolar solvents) added with BSA•The standard solutions are in a wide range of concentrations.•The preparation has a quality control procedure based on direct analysis of bilirubin UV-VIS spectra or fluorescence emission of the its complex with the recombinant fusion protein HELP-UnaG (HUG).
Keywords: Bilirubin standards; Methanol; Physiological solutions; Preparation of nanomolar aqueous bilirubin standard solutions; Stability.
© 2025 The Authors. Published by Elsevier B.V.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
-
- Brodersen R. Binding of bilirubin to albumin. CRC Crit. Rev. Clin. Lab. Sci. 1980;11:305–399. - PubMed
-
- Hulzebos C.V., Vitek L., Coda Zabetta C.D., Dvořák A., Schenk P., van der Hagen E.A.E., Cobbaert C., Tiribelli C. Diagnostic methods for neonatal hyperbilirubinemia: benefits, limitations, requirements, and novel developments. Pediatr. Res. 2021;90:277–283. doi: 10.1038/s41390-021-01546-y. - DOI - PubMed
LinkOut - more resources
Full Text Sources