Chemical Composition of Cynanchum auriculatum Royle Ex Wight and Its Potential Role in Ameliorating Colitis
- PMID: 39830908
- PMCID: PMC11742642
- DOI: 10.1002/fsn3.4764
Chemical Composition of Cynanchum auriculatum Royle Ex Wight and Its Potential Role in Ameliorating Colitis
Abstract
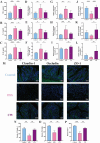
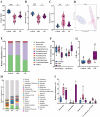
Cynanchum auriculatum Royle ex Wight, commonly known as "Baishouwu," has been traditionally used in China for its medicinal and dietary benefits. Despite its long history of use, the potential therapeutic effects of C. auriculatum in the treatment of colitis have not been fully investigated. This study aims to evaluate the effects of the water extract of C. auriculatum root on colitis and elucidate its potential mechanisms of action. The water extract of C. auriculatum root (CW) was prepared and characterized using UPLC-Q-TOF-MS, identifying thirty-two distinct compounds, including saponins, organic acids, fatty acid derivatives, and alkaloids. The therapeutic efficacy of CW was assessed in a colitis mouse model. CW significantly alleviated colitis symptoms, evidenced by increased colon length, reduced disease activity indices, and decreased colon tissue damage. CW reduced colonic inflammatory cytokine production and enhanced the expression of tight junction proteins, including claudin-1, occludin, and ZO-1, thereby strengthening intestinal barrier integrity. Additionally, CW modulated the gut microbiota by increasing microbial diversity, promoting beneficial Lactobacillus growth, reducing pathogenic Pseudomonas levels, and enhancing short-chain fatty acid production. The results suggest that CW exhibits significant therapeutic potential in the management of colitis by attenuating inflammation, restoring gut barrier function, and modulating the gut microbiota. These findings provide a basis for further exploration of C. auriculatum as a functional food for prevention and treatment of colitis.
Keywords: Cynanchum auriculatum; chemical composition; colitis; gut microbiota; short‐chain fatty acids.
© 2025 The Author(s). Food Science & Nutrition published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Comparative sub-chronic toxicity studies in rats of two indistinguishable herbal plants, Cynanchum wilfordii (Maxim.) Hemsley and Cynanchum auriculatum Royle ex Wight.Food Sci Biotechnol. 2022 Apr 19;31(6):759-766. doi: 10.1007/s10068-022-01072-5. eCollection 2022 Jun. Food Sci Biotechnol. 2022. PMID: 35646417 Free PMC article.
-
Polysaccharides from Cynanchum auriculatum Royle ex Wight ameliorate symptoms of hyperglycemia by regulating gut microbiota in type 2 diabetes mellitus mice.Int J Biol Macromol. 2025 Apr;299:139878. doi: 10.1016/j.ijbiomac.2025.139878. Epub 2025 Jan 14. Int J Biol Macromol. 2025. PMID: 39818385
-
Morphology, structural and physicochemical properties of starch from the root of Cynanchum auriculatum Royle ex Wight.Int J Biol Macromol. 2016 Dec;93(Pt A):107-116. doi: 10.1016/j.ijbiomac.2016.08.063. Epub 2016 Aug 24. Int J Biol Macromol. 2016. PMID: 27565292
-
Cynanchum bungei Decne and its two related species for "Baishouwu": A review on traditional uses, phytochemistry, and pharmacological activities.J Ethnopharmacol. 2019 Oct 28;243:112110. doi: 10.1016/j.jep.2019.112110. Epub 2019 Jul 24. J Ethnopharmacol. 2019. PMID: 31351190 Review.
-
Cynanchum auriculatum Royle ex Wight., Cynanchum bungei Decne. and Cynanchum wilfordii (Maxim.) Hemsl.: Current Research and Prospects.Molecules. 2021 Nov 23;26(23):7065. doi: 10.3390/molecules26237065. Molecules. 2021. PMID: 34885647 Free PMC article. Review.
References
-
- Chai, Z. , Huang W., Zhao X., Wu H., Zeng X., and Li C.. 2018. “Preparation, Characterization, Antioxidant Activity and Protective Effect Against Cellular Oxidative Stress of Polysaccharide From Cynanchum Auriculatum Royle ex Wight.” International Journal of Biological Macromolecules 119: 1068–1076. 10.1016/j.ijbiomac.2018.08.024. - DOI - PubMed
LinkOut - more resources
Full Text Sources

