Immunogenicity and vaccine efficacy of Actinobacillus pleuropneumoniae-derived extracellular vesicles as a novel vaccine candidate
- PMID: 39831520
- PMCID: PMC11749362
- DOI: 10.1080/21505594.2025.2453818
Immunogenicity and vaccine efficacy of Actinobacillus pleuropneumoniae-derived extracellular vesicles as a novel vaccine candidate
Abstract
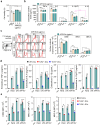
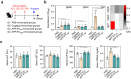
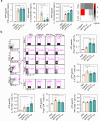
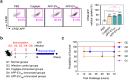
Actinobacillus pleuropneumoniae (APP) is a significant pathogen in the swine industry, leading to substantial economic losses and highlighting the need for effective vaccines. This study evaluates the potential of APP-derived extracellular vesicles (APP-EVs) as a vaccine candidate compared to the commercial Coglapix vaccine. APP-EVs, isolated using tangential flow filtration (TFF) and cushioned ultracentrifugation, exhibited an average size of 105 nm and a zeta potential of -17.4 mV. These EVs demonstrated stability under external stressors, such as pH changes and enzymatic exposure and were found to contain 86 major metabolites. Additionally, APP-EVs induced dendritic cell (DC) maturation in a Toll-like receptor 4 (TLR4)-dependent manner without cytotoxicity. APP-EVs predominantly elicited Th1-mediated IgG responses in immunized mice without significant liver and kidney toxicity. Contrarily, unlike Coglapix, which induced stronger Th2-mediated responses and notable toxicity. In addition, APP-EVs triggered APP-specific Th1, Th17, and cytotoxic T lymphocyte (CTL) responses and promoted the activation of multifunctional T-cells. Notably, APP-EV immunization enhanced macrophage phagocytosis and improved survival rates in mice challenged with APP infection compared to those treated with Coglapix. These findings suggest that APP-EVs are promising vaccine candidates, capable of inducing potent APP-specific T-cell responses, particularly Th1, Th17, CTL, and multifunctional T-cells, thereby enhancing the protective immune response against APP infection.
Keywords: Actinobacillus pleuropneumoniae; Th1-dominant cellullar immunity; Th1-dominant humoral immunity; extracellular vesicle; immunogenicity; pre-exposure vaccine.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures


Similar articles
-
Immunization of pigs with Actinobacillus pleuropneumonia live attenuated (gene-deleted) vaccine HB04M intramuscularly or intranasally exhibits remarkably rapid protection against heterologous strain challenge.BMC Vet Res. 2025 Jul 8;21(1):450. doi: 10.1186/s12917-025-04895-6. BMC Vet Res. 2025. PMID: 40629392 Free PMC article.
-
Immunogenicity and protective efficacy of ApxIA and ApxIIA DNA vaccine against Actinobacillus pleuropneumoniae lethal challenge in murine model.Vaccine. 2009 Jul 23;27(34):4565-70. doi: 10.1016/j.vaccine.2009.05.058. Epub 2009 Jun 9. Vaccine. 2009. PMID: 19520199
-
DNA vaccine encoding type IV pilin of Actinobacillus pleuropneumoniae induces strong immune response but confers limited protective efficacy against serotype 2 challenge.Vaccine. 2011 Oct 13;29(44):7740-6. doi: 10.1016/j.vaccine.2011.07.127. Epub 2011 Aug 9. Vaccine. 2011. PMID: 21835218
-
Immunogenicity and seroefficacy of pneumococcal conjugate vaccines: a systematic review and network meta-analysis.Health Technol Assess. 2024 Jul;28(34):1-109. doi: 10.3310/YWHA3079. Health Technol Assess. 2024. PMID: 39046101 Free PMC article.
-
New trends in innovative vaccine development against Actinobacillus pleuropneumoniae.Vet Microbiol. 2018 Apr;217:66-75. doi: 10.1016/j.vetmic.2018.02.028. Epub 2018 Mar 6. Vet Microbiol. 2018. PMID: 29615259 Review.
Cited by
-
Nanomaterial Adjuvants for Veterinary Vaccines: Mechanisms and Applications.Research (Wash D C). 2025 Jul 8;8:0761. doi: 10.34133/research.0761. eCollection 2025. Research (Wash D C). 2025. PMID: 40636132 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
