Protective immunity induced by a novel P1 adhesin C-terminal anchored mRNA vaccine against Mycoplasma pneumoniae infection in BALB/c mice
- PMID: 39831768
- PMCID: PMC11878037
- DOI: 10.1128/spectrum.02140-24
Protective immunity induced by a novel P1 adhesin C-terminal anchored mRNA vaccine against Mycoplasma pneumoniae infection in BALB/c mice
Abstract
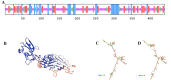
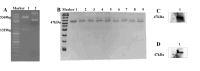
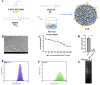
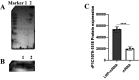
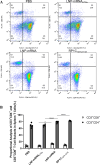
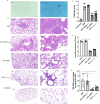
Mycoplasma pneumoniae (Mp), a unique pathogen devoid of a cell wall, is naturally impervious to penicillin antibiotics. This bacterium is the causative agent of M. pneumoniae pneumonia, an acute pulmonary affliction marked by interstitial lung damage. Non-macrolide medications may have potential adverse effects on the developmental trajectory of children, thereby establishing macrolides as the preferred treatment for M. pneumoniae in pediatric patients. However, the emergence of macrolide-resistant and multidrug-resistant strains of M. pneumoniae presents significant challenges to clinical management and public health. Vaccines, particularly those based on mRNA technology, are regarded as a promising avenue for preventing and controlling M. pneumoniae infections due to their inherent safety, immunogenicity, and adaptability. Our research delves into the P1 adhesin of M. pneumoniae, a protein that binds to host cell receptors with its immunodominant epitopes located at the carboxyl terminus, known to provoke robust immune responses and pulmonary inflammation. We have developed an mRNA vaccine harnessing this dominant antigenic epitope and assessed its protective immunity in BALB/c mice against M. pneumoniae infection. The vaccine elicited potent humoral and cellular immune responses, effectively diminishing inflammation. It notably decreased IL-6 levels in the lungs of infected mice and concurrently elevated IL-4, IL-10, and IFN-γ levels post-immunization. The vaccine also reduced pathological changes in the lungs and the M. pneumoniae DNA copy numbers in the infected animals. Collectively, these findings underscore the mRNA vaccine's remarkable immunogenicity and protective potential against M. pneumoniae infections, offering valuable insights for the development of mRNA vaccines targeting mycoplasma infections.IMPORTANCEM. pneumoniae, a bacteria without a cell wall, is known for causing pneumonia and is resistant to penicillin. The increasing prevalence of macrolide-resistant strains has complicated treatment options, emphasizing the need for new strategies. Our research explores an mRNA vaccine candidate that targets the P1 adhesin of M. pneumoniae, a protein critical for the bacteria's interaction with host cells. In a mouse model, this vaccine has shown potential by inducing immune responses and suggesting a possible reduction in inflammation, as indicated by changes in cytokine levels and lung pathology. While further research is required, the vaccine's preliminary results hint at a potential new direction in managing mycoplasma infections, offering a promising avenue for future therapeutic development. This study contributes to the ongoing search for effective preventive measures against M. pneumoniae.
Keywords: Mycoplasma pneumoniae; P1 adhesin; dominant antigenic epitope; lipid nanoparticle; mRNA vaccine; protective immunity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


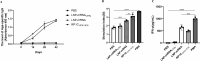
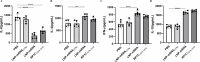
References
MeSH terms
Substances
Grants and funding
- 2023JJ40101/HSTD | Natural Science Foundation of Hunan Province ()
- 32370206/MOST | National Natural Science Foundation of China (NSFC)
- 81902077/National Youth Science Foundation (NYSF)
- 2020JJ5476/HSTD | Science Fund for Distinguished Young Scholars of Hunan Province ()
- A2024592/Health Department of Guangdong Province | Medical Science and Technology Foundation of Guangdong Province (Science and Technology Foundation of Guangdong Province)
LinkOut - more resources
Full Text Sources
Other Literature Sources

