Activation of the COX-2/mPGES-1/PGE-2 cascade through the NLRP3 inflammasome contributes to Angiostrongylus cantonensis-induced eosinophilic meningoencephalitis
- PMID: 39832004
- PMCID: PMC11753341
- DOI: 10.1007/s00436-025-08454-8
Activation of the COX-2/mPGES-1/PGE-2 cascade through the NLRP3 inflammasome contributes to Angiostrongylus cantonensis-induced eosinophilic meningoencephalitis
Abstract
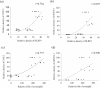
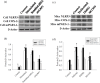
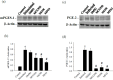
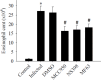
Prostaglandin E2 (PGE-2) is synthesised by cyclooxygenase-2 (COX-2) and microsomal prostaglandin E synthase 1 (mPGES-1). PGE-2 exhibits pro-inflammatory properties in inflammatory conditions. However, there remains limited understanding of the COX-2/mPGES-1/PGE-2 pathway in Angiostrongylus cantonensis-induced meningoencephalitis. This study revealed several key findings regarding the activation of the COX-2/mPGES-1/PGE-2 pathway and its correlation with eosinophilic meningoencephalitis induced by A. cantonensis infection. Immunostaining revealed an increase in the expression of COX-2 and mPGES-1 in the subarachnoid space and glial cells compared to control subjects. Inhibition of the NLRP3 inflammasome by small interfering RNA (siRNA) blocked extracellular secretory proteins (ESPs) stimulated COX-2, mPGES-1 and PGE-2 in microglia. MCC950, an NLRP3 inhibitor, inhibited the levels of the COX-2, mPGES-1, and PGE-2 proteins induced by A. cantonensis in mice. Treatment of mice infected with A. cantonensis with the COX-2 inhibitor NS398 significantly reduced the levels of mPGES-1, PGE-2, and matrix metalloproteinase-9 (MMP-9) levels. Similarly, the mPGES-1 inhibitor MF63 significantly reduced PGE-2 and MMP-9 levels in A. cantonensis-infected mice. Administration of MCC950, NS398, or MF63 resulted in marked attenuation of blood-brain barrier (BBB) permeability and eosinophil counts in A. cantonensis-infected mice. These findings highlight the critical role of the COX-2/mPGES-1/PGE-2 pathway and its regulation by the NLRP3 inflammasome in the pathogenesis of eosinophilic meningoencephalitis induced by A. cantonensis infection. Furthermore, pharmacological interventions targeting this pathway, such as MCC950, NS398, and MF63, show promising therapeutic potential in mitigating associated inflammatory responses and disruption of the BBB. The results indicate that blocking NLRP3 using pharmacological (MCC950) and gene silencing (siNLRP3) methods emphasised the crucial involvement of NLRP3 in the COX-2/mPGES-1/PGE-2 pathway. This suggests that the activation of the COX-2/mPGES-1/PGE-2 axis in response to A. cantonensis infection may be mediated through a mechanism involving the NLRP3 inflammasome.
Keywords: Angiostrongylus cantonensis; Cyclooxygenase-2; Inflammasome; Meningoencephalitis; Prostaglandin E2.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approval: This study received approval from the Institutional Animal Care and Use Committee of Chung-Shan Medical University, and all procedures were conducted following institutional guidelines for animal experiments. The manuscript does not include any clinical or patient data. Consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures






Similar articles
-
Activation of COX-2/mPGES-1/PGE2 Cascade via NLRP3 Inflammasome Contributes to Albumin-Induced Proximal Tubule Cell Injury.Cell Physiol Biochem. 2017;42(2):797-807. doi: 10.1159/000478070. Epub 2017 Jun 19. Cell Physiol Biochem. 2017. PMID: 28628921
-
Matrix metalloproteinase-9 leads to blood-brain barrier leakage in mice with eosinophilic meningoencephalitis caused by Angiostrongylus cantonensis.Acta Trop. 2014 Dec;140:141-50. doi: 10.1016/j.actatropica.2014.08.015. Epub 2014 Aug 23. Acta Trop. 2014. PMID: 25158284
-
Curative effects and mechanisms of AG1296 and LY294002 co-therapy in Angiostrongylus cantonensis-induced neurovascular unit dysfunction and eosinophilic meningoencephalitis.J Microbiol Immunol Infect. 2024 Aug;57(4):647-659. doi: 10.1016/j.jmii.2024.05.012. Epub 2024 May 30. J Microbiol Immunol Infect. 2024. PMID: 38839542
-
[Advances in pathogenic mechanisms of Angiostrongylus cantonensis infection].Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi. 2019 Mar 16;31(1):98-104. doi: 10.16250/j.32.1374.2018305. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi. 2019. PMID: 31016931 Review. Chinese.
-
Membrane prostaglandin E synthase-1: a novel therapeutic target.Pharmacol Rev. 2007 Sep;59(3):207-24. doi: 10.1124/pr.59.3.1. Pharmacol Rev. 2007. PMID: 17878511 Review.
References
-
- Aid S, Silva AC, Candelario-Jalil E, Choi SH, Rosenberg GA, Bosetti F (2010) Cyclooxygenase-1 and -2 differentially modulate lipopolysaccharide-induced blood-brain barrier disruption through matrix metalloproteinase activity. J Cereb Blood Flow Metab 30:370–380. 10.1038/jcbfm.2009.223 - PMC - PubMed
-
- Blais V, Turrin NP, Rivest S (2005) Cyclooxygenase 2 (COX-2) inhibition increases the inflammatory response in the brain during systemic immune stimuli. J Neurochem 95:1563–1574. 10.1111/j.1471-4159.2005.03480.x - PubMed
-
- Candelario-Jalil E, Gonzalez-Falcon A, Garcia-Cabrera M, Leon OS, Fiebich BL (2007) Post-ischaemic treatment with the cyclooxygenase-2 inhibitor nimesulide reduces blood-brain barrier disruption and leukocyte infiltration following transient focal cerebral ischaemia in rats. J Neurochem 100:1108–1120 - PubMed
-
- Chen AC, Shyu LY, Hsin YL, Chen KM, Lai SC (2017) Resveratrol relieves Angiostrongylus cantonensis - induced meningoencephalitis by activating sirtuin-1. Acta Trop 173:76–84. 10.1016/j.actatropica.2017.05.023 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

