Timing of exercise differentially impacts adipose tissue gain in male adolescent rats
- PMID: 39832562
- PMCID: PMC11810837
- DOI: 10.1016/j.molmet.2025.102100
Timing of exercise differentially impacts adipose tissue gain in male adolescent rats
Erratum in
-
Corrigendum to "Timing of exercise differentially impacts adipose tissue gain in male adolescent rats" [Mol Metabol 93 (2025) 102100, 1-14].Mol Metab. 2025 Mar;93:102110. doi: 10.1016/j.molmet.2025.102110. Epub 2025 Feb 11. Mol Metab. 2025. PMID: 39929253 Free PMC article. No abstract available.
Abstract
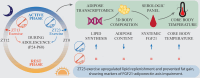
Objective: Circadian rhythms of metabolic, hormonal, and behavioral fluctuations and their alterations can impact health. An important gap in knowledge in the field is whether the time of the day of exercise and the age of onset of exercise exert distinct effects at the level of whole-body adipose tissue and body composition. The goal of the present study was to determine how exercise at different times of the day during adolescence impacts the adipose tissue transcriptome and content in a rodent model.
Methods: Rats were subjected to one of four conditions during their adolescence: early active phase control or exercise (EAC or EAE; ZT13), and late active phase control or exercise (LAC or LAE; ZT23). The effects of exercise timing were assessed at the level of subcutaneous and visceral adipose tissue transcriptome, body composition, hypothalamic expression of orexigenic and anorexigenic genes, blood serum markers and 24-hour core body temperature patterns.
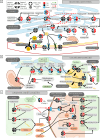
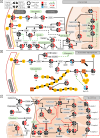
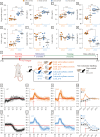
Results: We found that late active phase exercise (ZT23) greatly upregulated pathways of lipid synthesis, glycolysis and NADH shuttles in LAE rats, compared to LAC or EAE. Conversely, LAE rats showed notably lower content of adipose tissue. In addition, LAE rats showed signs of impaired FGF21-adiponectin axis compared to other groups.
Conclusions: Finally, LAE rats showed higher post-exercise core body temperature compared to other groups. Our results thus indicate that our exercise protocol induced an unusual effect characterized by enhanced lipid synthesis but reduced adipose tissue content in late active phase but not early active phase exercise during adolescence.
Keywords: Adipose tissue; Adolescence; Body composition; Circadian system; Forced exercise; Rodent; Transcriptomics.
Copyright © 2025 The Author(s). Published by Elsevier GmbH.. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
-
- Zhang Z., Xin H., Li M.-D. Circadian rhythm of lipid metabolism in health and disease. Small Methods. 2020;4 doi: 10.1002/smtd.201900601. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

