Unlocking novel T cell-based immunotherapy for hepatocellular carcinoma through neoantigen-driven T cell receptor isolation
- PMID: 39832892
- PMCID: PMC12611799
- DOI: 10.1136/gutjnl-2024-334148
Unlocking novel T cell-based immunotherapy for hepatocellular carcinoma through neoantigen-driven T cell receptor isolation
Abstract
Background: Tumour-infiltrating T cells can mediate both antitumour immunity and promote tumour progression by creating an immunosuppressive environment. This dual role is especially relevant in hepatocellular carcinoma (HCC), characterised by a unique microenvironment and limited success with current immunotherapy.
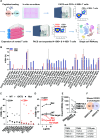
Objective: We evaluated T cell responses in patients with advanced HCC by analysing tumours, liver flushes and liver-draining lymph nodes, to understand whether reactive T cell populations could be identified despite the immunosuppressive environment.
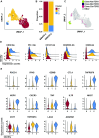
Design: T cells isolated from clinical samples were tested for reactivity against predicted neoantigens. Single-cell RNA sequencing was employed to evaluate the transcriptomic and proteomic profiles of antigen-experienced T cells. Neoantigen-reactive T cells expressing 4-1BB were isolated and characterised through T-cell receptor (TCR)-sequencing.
Results: Bioinformatic analysis identified 542 candidate neoantigens from seven patients. Of these, 78 neoantigens, along with 11 hotspot targets from HCC driver oncogenes, were selected for ex vivo T cell stimulation. Reactivity was confirmed in co-culture assays for 14 targets, with most reactive T cells derived from liver flushes and lymph nodes. Liver flush-derived T cells exhibited central memory and effector memory CD4+ with cytotoxic effector profiles. In contrast, tissue-resident memory CD4+ and CD8+ T cells with an exhausted profile were primarily identified in the draining lymph nodes.
Conclusion: These findings offer valuable insights into the functional profiles of neoantigen-reactive T cells within and surrounding the HCC microenvironment. T cells isolated from liver flushes and tumour-draining lymph nodes may serve as a promising source of reactive T cells and TCRs for further use in immunotherapy for HCC.
Keywords: ANTIGENS; HEPATOCELLULAR CARCINOMA; IMMUNOTHERAPY; T LYMPHOCYTES; T-CELL RECEPTOR.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: None declared.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
