Enhanced effect of the immunosuppressive soluble HLA-G2 homodimer by site-specific PEGylation
- PMID: 39833185
- PMCID: PMC11756389
- DOI: 10.1038/s41598-024-85072-x
Enhanced effect of the immunosuppressive soluble HLA-G2 homodimer by site-specific PEGylation
Abstract
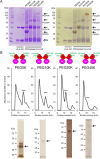
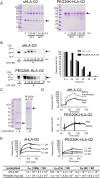
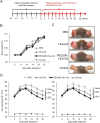
Human leukocyte antigen (HLA)-G is a nonclassical HLA class I molecule that has an immunosuppressive effect mediated by binding to immune inhibitory leukocyte immunoglobulin-like receptors (LILR) B1 and LILRB2. A conventional HLA-G isoform, HLA-G1, forms a heterotrimeric complex composed of a heavy chain (α1-α3 domains), β2-microglobulin (β2m) and a cognate peptide. One of the other isoforms, HLA-G2, lacks a α2 domain or β2m to form a nondisulfide-linked homodimer, and its ectodomain specifically binds to LILRB2 expressed in human monocytes, macrophages, and dendritic cells. The administration of the ectodomain of HLA-G2, designated the soluble HLA-G2 homodimer, showed significant immunosuppressive effects in mouse models of rheumatoid arthritis and systemic lupus erythematosus, presumably by binding to a mouse ortholog of LILRB2, paired immunoglobulin-like receptor B. However, the refolded soluble HLA-G2 homodimer used in these studies tends to aggregate and degrade; thus, its stability for clinical use has been a concern. In the present study, we improved the stability of the refolded soluble HLA-G2 homodimer via a site-directed PEGylation method. PEGylation at an original free cysteine residue, Cys42, resulted in increased lyophilization and thermal and serum stability. Furthermore, the PEGylated soluble HLA-G2 homodimer could better suppress atopic symptoms in mice than the non-PEGylated homodimer. These results suggest that PEGylated soluble HLA-G2 homodimers could be candidates for immunosuppressive biologics that specifically target LILRB2-positive myelomonocytic antigen-presenting cells.
Keywords: HLA-G2; Immune checkpoint; Immunosuppressive; LILR; PEGylation.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Consent for publication: All the authors read and approved the final manuscript.
Figures




References
-
- Kamishikiryo, J. & Maenaka, K. HLA-G molecule. Curr. Pharm. Des.15, 3318–3324. 10.2174/138161209789105153 (2009). - PubMed
-
- Kuroki, K. et al. Cutting edge: Class II-like structural features and strong receptor binding of the nonclassical HLA-G2 isoform homodimer. J. Immunol.198, 3399–3403. 10.4049/jimmunol.1601296 (2017). - PubMed
-
- Bahri, R. et al. Soluble HLA-G inhibits cell cycle progression in human alloreactive T lymphocytes. J. Immunol.176, 1331–1339. 10.4049/jimmunol.176.3.1331 (2006). - PubMed
-
- Gonen-Gross, T. et al. Complexes of HLA-G protein on the cell surface are important for leukocyte Ig-like receptor-1 function. J. Immunol.171, 1343–1351. 10.4049/jimmunol.171.3.1343 (2003). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- JP23770102/Japan Society for the Promotion of Science
- JP16J05871/Japan Society for the Promotion of Science
- JP20H05873/Japan Society for the Promotion of Science
- research grant/Naito Science and Engineering Foundation
- JP22ama121037, JP223fa627005, JP22gm1810004/Japan Agency for Medical Research and Development
LinkOut - more resources
Full Text Sources
Research Materials

