The efficacy and safety of patient-controlled intravenous analgesia with esketamine after total hip arthroplasty: a randomized controlled trial
- PMID: 39833672
- PMCID: PMC11744849
- DOI: 10.1186/s12871-025-02894-6
The efficacy and safety of patient-controlled intravenous analgesia with esketamine after total hip arthroplasty: a randomized controlled trial
Abstract
Purpose: To evaluate the efficacy and safety of esketamine-based patient-controlled intravenous analgesia following total hip arthroplasty.
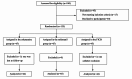
Methods: A total of 135 total hip arthroplasty patients were randomly assigned to one of the three treatment groups: esketamine, sufentanil or continuous fascia iliaca compartment block (FICB) group. The primary endpoint was the postoperative visual analogue scale (VAS) pain scores at rest and on movement. Secondary endpoints included preoperative 1-day and postoperative 7-day Self-Rating Anxiety Scale (SAS) and Self-Rating Depression Scale (SDS) scores, the satisfaction of patients and surgeons, postoperative 1-month and 3-month Harris function scores, the incidence of adverse reactions.
Results: At 48 h post-surgery, the VAS pain scores in the esketamine and FICB groups at rest and on movement were significantly lower than those in the sufentanil group (P < 0.05). The satisfaction of patients in the esketamine group was higher than that in the sufentanil and FICB groups (P = 0.014). The satisfaction of surgeons in the esketamine and FICB groups was higher than that in the sufentanil group (P = 0.002). At postoperative day 7, the SAS scores and SDS scores in the esketamine group were significantly lower than those in the sufentanil and FICB groups (P < 0.05). Compared with the sufentanil group, the postoperative nausea and vomiting, dizziness and total adverse reactions in the esketamine group and FICB group were lower (P < 0.05).
Conclusion: Patient-controlled intravenous analgesia with esketamine has the potential to provide good postoperative analgesia for total hip arthroplasty patients, reduce the incidence of adverse reactions after the operation, improve the satisfaction of patients and surgeons, and significantly improve patients' postoperative mood.
Trial registration: ChiCTR2300069632 ( https://www.chictr.org.cn/ ) (March 22th, 2023).
Keywords: Esketamine; Fascia Iliaca compartment block; Patient controlled intravenous analgesia; Postoperative pain; Total hip arthroplasty.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approval and consent to Participate: This study was approved by the Research Ethics Committees of Gansu Provincial Hospital (Ethical code: 2022 − 423) and was registered at the Chinese Clinical Trial Registry ( https://www.chictr.org.cn/ , identifier: ChiCTR2300069632) on March 22th, 2023. Written informed consent was obtained from the patients to publish this article in accordance with the journal’s patient consent policy. The study was conducted in accordance with the Declarations of Helsinki, and adhered to the CONSORT guidelines. Consent for publication: N/A. Competing interests: The authors declare no competing interests.
Figures
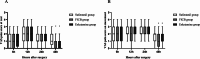
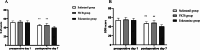
Similar articles
-
Safety and efficacy of low-dose esketamine weakly opioidized anesthesia in elderly patients with lumbar spinal stenosis undergoing surgery: a prospective, double-blind randomized controlled trial.BMC Anesthesiol. 2025 Feb 5;25(1):57. doi: 10.1186/s12871-025-02908-3. BMC Anesthesiol. 2025. PMID: 39910473 Free PMC article. Clinical Trial.
-
Comparative analysis of continuous pericapsular nerve group block and supra-inguinal fascia iliaca compartment block for postoperative analgesia in total hip arthroplasty: a randomized controlled trial.J Orthop Surg Res. 2025 Aug 6;20(1):729. doi: 10.1186/s13018-025-06055-w. J Orthop Surg Res. 2025. PMID: 40770360 Free PMC article. Clinical Trial.
-
Esketamine use for primary intelligent analgesia in adults with severe burns: A double-blind randomized trial with effects on analgesic efficacy, gastrointestinal function and mental state.Burns. 2024 Dec;50(9):107187. doi: 10.1016/j.burns.2024.06.004. Epub 2024 Jun 22. Burns. 2024. PMID: 39317541 Clinical Trial.
-
Patient-controlled analgesia with remifentanil versus alternative parenteral methods for pain management in labour.Cochrane Database Syst Rev. 2017 Apr 13;4(4):CD011989. doi: 10.1002/14651858.CD011989.pub2. Cochrane Database Syst Rev. 2017. PMID: 28407220 Free PMC article.
-
Epidural analgesia for pain relief following hip or knee replacement.Cochrane Database Syst Rev. 2003;2003(3):CD003071. doi: 10.1002/14651858.CD003071. Cochrane Database Syst Rev. 2003. PMID: 12917945 Free PMC article.
References
-
- Gao Y, Tan H, Sun R, Zhu J. Fascia Iliaca compartment block reduces pain and opioid consumption after total hip arthroplasty: a systematic review and meta-analysis. Int J Surg. 2019;65:70–9. - PubMed
-
- Zachodnik J, Geisler A. Short-term and Long-Term Pain after total hip arthroplasty: a prospective cohort study. Pain Manag Nurs. 2022;23(2):225–30. - PubMed
-
- Gaffney CJ, Pelt CE, Gililland JM, Peters CL. Perioperative Pain Management in hip and knee arthroplasty. Orthop Clin North Am. 2017;48(4):407–19. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

