Advances in nucleic acid-based cancer vaccines
- PMID: 39833784
- PMCID: PMC11748563
- DOI: 10.1186/s12929-024-01102-w
Advances in nucleic acid-based cancer vaccines
Abstract
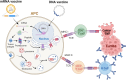
Nucleic acid vaccines have emerged as crucial advancements in vaccine technology, particularly highlighted by the global response to the COVID-19 pandemic. The widespread administration of mRNA vaccines against COVID-19 to billions globally marks a significant milestone. Furthermore, the approval of an mRNA vaccine for Respiratory Syncytial Virus (RSV) this year underscores the versatility of this technology. In oncology, the combination of mRNA vaccine encoding neoantigens and immune checkpoint inhibitors (ICIs) has shown remarkable efficacy in eliciting protective responses against diseases like melanoma and pancreatic cancer. Although the use of a COVID-19 DNA vaccine has been limited to India, the inherent stability at room temperature and cost-effectiveness of DNA vaccines present a viable option that could benefit developing countries. These advantages may help DNA vaccines address some of the challenges associated with mRNA vaccines. Currently, several trials are exploring the use of DNA-encoded neoantigens in combination with ICIs across various cancer types. These studies highlight the promising role of nucleic acid-based vaccines as the next generation of immunotherapeutic agents in cancer treatment. This review will delve into the recent advancements and current developmental status of both mRNA and DNA-based cancer vaccines.
Keywords: Cancer vaccine; DNA vaccine; Neoantigen; mRNA vaccine.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare that they have no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

