Microglial double stranded DNA accumulation induced by DNase II deficiency drives neuroinflammation and neurodegeneration
- PMID: 39833906
- PMCID: PMC11745000
- DOI: 10.1186/s12974-025-03333-6
Microglial double stranded DNA accumulation induced by DNase II deficiency drives neuroinflammation and neurodegeneration
Abstract
Background: Deoxyribonuclease 2 (DNase II) is pivotal in the clearance of cytoplasmic double stranded DNA (dsDNA). Its deficiency incurs DNA accumulation in cytoplasm, which is a hallmark of multiple neurodegenerative diseases. Our previous study showed that neuronal DNase II deficiency drove tau hyperphosphorylation and neurodegeneration (Li et al., Transl Neurodegener 13:39, 2024). Although it has been verified that DNase II participates in type I interferons (IFN-I) mediated autoinflammation and senescence in peripheral systems, the role of microglial DNase II in neuroinflammation and neurodegenerative diseases such as Alzheimer's disease (AD) is still unknown.
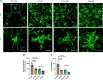
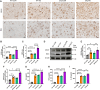
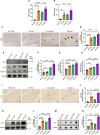
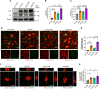
Methods: The levels of microglial DNase II in triple transgenic AD mice (3xTg-AD) were measured by immunohistochemistry. The cognitive performance of microglial DNase II deficient WT and AD mice was determined using the Morris water maze test, Y-maze test, novel object recognition test and open filed test. To investigate the impact of microglial DNase II deficiency on microglial morphology, cyclic GMP-AMP synthase (cGAS)-stimulator of interferon genes (STING) pathway and IFN-I pathway, neuroinflammation, synapses loss, amyloid pathology and tauopathy, the levels of cGAS-STING and IFN-I pathway related protein, gliosis and proinflammatory cytokines, synaptic protein, complement protein, Aβ levels, phosphorylated tau in the brains of the microglial DNase II deficient WT and AD mice were evaluated by immunolabeling, immunoblotting, q-PCR or ELISA.
Results: We found that the levels of DNase II were significantly decreased in the microglia of 3xTg-AD mice. Microglial DNase II deficiency altered microglial morphology and transcriptional signatures, activated the cGAS-STING and IFN-I pathway, initiated neuroinflammation, led to synapse loss via complement-dependent pathway, increased Aβ levels and tauopathy, and induced cognitive decline.
Conclusions: Our study shows the effect of microglial DNase II deficiency and cytoplasmic accumulated dsDNA on neuroinflammation, and reveals the initiatory mechanism of AD pathology, suggesting that DNase II is a potential target for neurodegenerative diseases.
Keywords: Alzheimer's disease (AD); DNase II; Double stranded DNA (dsDNA); Neuroinflammation; Type I interferons pathway; cGAS-STING pathway.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All animal experiments were performed in accordance with the China Public Health Service Guide for the Care and Use of Laboratory Animals. Experiments involving mice and protocols were approved by the Institutional Animal Care and Use Committee of Tsinghua University. Authors are responsible for correctness of the statements provided in the manuscript. Consent for publication: All authors have reviewed the final manuscript and consent to publication. Competing interests: The authors declare no competing interests.
Figures




References
-
- (2023) 2023 Alzheimer's disease facts and figures. Alzheimer Dement 19:1598–1695 - PubMed
-
- Heppner FL, Ransohoff RM, Becher B. Immune attack: the role of inflammation in Alzheimer disease. Nat Rev Neurosci. 2015;16:358–72. - PubMed
-
- Perlmutter D. Preventing Alzheimer’s disease. J Am Coll Nutr. 2016;35:732–3. - PubMed
-
- Du L, Zhang Y, Chen Y, Zhu J, Yang Y, Zhang HL. Role of microglia in neurological disorders and their potentials as a therapeutic target. Mol Neurobiol. 2017;54:7567–84. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

