CD36 enrichment in HER2-positive mesenchymal stem cells drives therapy refractoriness in breast cancer
- PMID: 39833955
- PMCID: PMC11744895
- DOI: 10.1186/s13046-025-03276-z
CD36 enrichment in HER2-positive mesenchymal stem cells drives therapy refractoriness in breast cancer
Erratum in
-
Correction: CD36 enrichment in HER2-positive mesenchymal stem cells drives therapy refractoriness in breast cancer.J Exp Clin Cancer Res. 2025 May 30;44(1):165. doi: 10.1186/s13046-025-03426-3. J Exp Clin Cancer Res. 2025. PMID: 40442724 Free PMC article. No abstract available.
Abstract
Background: Growing evidence shows that the reprogramming of fatty acid (FA) metabolism plays a key role in HER2-positive (HER2 +) breast cancer (BC) aggressiveness, therapy resistance and cancer stemness. In particular, HER2 + BC has been defined as a "lipogenic disease" due to the functional and bi-directional crosstalk occurring between HER2-mediated oncogenic signaling and FA biosynthesis via FA synthase activity. In this context, the functional role exerted by the reprogramming of CD36-mediated FA uptake in HER2 + BC poor prognosis and therapy resistance remains unclear. In this study, we aimed to elucidate whether enhanced CD36 in mesenchymal HER2 + cancer stem cells (CSCs) is directly involved in anti-HER2 treatment refractoriness in HER2 + BC and to design future metabolism-based approaches targeting both FA reprogramming and the "root" of cancer.
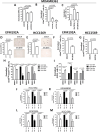
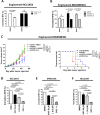
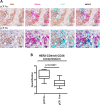
Methods: Molecular, biological and functional characterization of CD36-mediated FA uptake was investigated in HER2 + BC patients, cell lines, epithelial and mesenchymal CSCs. Cell proliferation was analyzed by SRB assay upon treatment with lapatinib, CD36 inhibitor, or Wnt antagonist/agonist. Engineered cell models were generated via lentivirus infection and transient silencing. CSC-like properties and tumorigenesis of HER2 + BC cells with or without CD36 depletion were examined by mammosphere forming efficiency assay, flow cytometry, cell sorting, ALDH activity assay and xenograft mouse model. FA uptake was examined by flow cytometry with FA BODIPY FL C16. Intratumor expression of CSC subsets was evaluated via multiplex immunostaining and immunolocalization analysis.
Results: Molecular data demonstrated that CD36 is significantly upmodulated on treatment in therapy resistant HER2 + BC patients and its expression levels in BC cells is correlated with FA uptake. We provided evidence of a consistent enrichment of CD36 in HER2 + epithelial-mesenchymal transition (EMT)-like CSCs from all tested resistant cell models that mechanistically occurs via Wnt signaling pathway activation. Consistently, both in vitro and in vivo dual blockade of CD36 and HER2 increased the anti-CSC efficacy of anti-HER2 drugs favoring the transition of the therapy resistant mesenchymal CSCs into therapy-sensitive mesenchymal-epithelial transition (MET)-like epithelial state. In addition, expression of CD36 in intratumor HER2 + mesenchymal CSCs is significantly associated with resistance to trastuzumab in HER2 + BC patients.
Conclusions: These results support the metabolo-oncogenic nature of CD36-mediated FA uptake in HER2 + therapy-refractory BC. Our study provides evidence that targeting CD36 might be an effective metabolic therapeutic strategy in the treatment of this malignancy.
Keywords: CD36; Cancer Stem Cells; Fatty Acid Uptake; HER2; Resistance to anti-HER2 therapy; Wnt pathway.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All procedures were performed in accordance with the Helsinki Declaration. The breast cancer biospecimens used for research consisted of leftover material from samples collected during standard surgical and medical approaches at INT. Samples were donated by patients to the Institutional BioBank for research purposes, and aliquots were allocated to this study after approval by the Institutional Review Board and a specific request to the Independent Ethics Committee of the INT (N. INT 170–20; Responsible Researcher Dr G. Pruneri). Animal experiments were approved by the Ethics Committee for Animal Experimentation of the INT according to institutional guidelines and the Italian Law (D-lgs 26/2014). In vivo experiments were approved by the Italian Ministry of Health (authorization numbers 1091/2020-PR and 74312.31.EXT.9; Resposible Researcher Dr S.M. Pupa). Consent for publication: Not applicable. Competing interests: F. Pietrantonio reports honoraria/consulting fees from Amgen, Merck-Serono, Sanofi, Ely Lilly, Bayer, Servier, Pierre-Fabre, AstraZeneca, BMS, MSD, and Organon and research grants from AstraZeneca, BMS, and Incyte. G. Pruneri reports honoraria from Eli Lilly, Roche Foundation One, Bayer, and Novartis. C. Vernieri reports research grants from Roche (to the Institution); honoraria as a speaker for educational events from Eli Lilly, Novartis, Accademia di Medicina, Fenix, and Istituto Gentili and for Advisory Board duties from Eli Lilly, Novartis, Menarini, Pfizer, and Daiichi Sankyo; and consulting fees from Eli Lilly. A. Vingiani reports honoraria from Roche. No disclosures were reported by the other authors.
Figures



References
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209–49. - PubMed
-
- Loibl S, Gianni L. HER2-positive breast cancer. Lancet. 2017;389:2415–29. - PubMed
-
- Vernieri C, Milano M, Brambilla M, Mennitto A, Maggi C, Cona MS, et al. Resistance mechanisms to anti-HER2 therapies in HER2-positive breast cancer: Current knowledge, new research directions and therapeutic perspectives. Crit Rev Oncol Hematol. 2019;139:53–66. - PubMed
-
- Hanahan D. Hallmarks of cancer: new dimensions. Cancer Discov. 2022;12:31–46. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

