Electroacupuncture Pretreatment Reduces Ischemic Brain Injury by Inhibiting the Lactate Production and Its Derived Protein Lactylation Formation
- PMID: 39834103
- PMCID: PMC11746925
- DOI: 10.1111/cns.70231
Electroacupuncture Pretreatment Reduces Ischemic Brain Injury by Inhibiting the Lactate Production and Its Derived Protein Lactylation Formation
Abstract
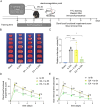
Aim: Given that electroacupuncture (EA) pretreatment inhibits lactate production and lactate-derived lysine lactation (Kla) aggravates ischemic brain injury, we aimed to investigate whether the formation of Kla protein is involved in EA pretreatment to alleviate ischemic brain injury.
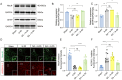
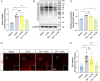
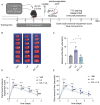
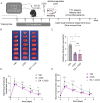
Methods: EA was performed on the Baihui acupoint (GV20) of male C57BL/6J mice before receiving the permanent middle cerebral artery occlusion (pMCAO) surgery. Western blot and immunofluorescent staining were used to observe neuronal survival, astrocyte activation, and protein Kla levels, and the lactate levels in ischemic brains were assayed with a commercial kit. TTC staining and neurological function scores are performed to evaluate the brain damage in mice.
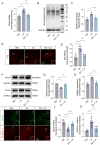
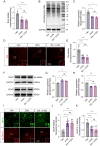
Results: We found that the increased lactate content and protein Kla levels were significantly decreased in ischemic brain tissue of mice after receiving EA pretreatment, and accompanied by the reduction of astrocyte activation and neuronal injury and death. Meantime, we found that EA pretreatment was effective in reversing the worsening of ischemic brain injury caused by lactate supplementation. However, EA pretreatment did not further reduce the lactate content and protein Kla levels and ameliorate brain injury in ischemic stroke mice after inhibition of glycolysis.
Conclusion: Our study reveals that EA pretreatment reduced ischemic brain damage by inhibiting lactate production and its derived protein Kla formation in mice with ischemic stroke.
Keywords: electroacupuncture pretreatment; glycolysis; ischemic stroke; lactate; lysine lactylation.
© 2025 The Author(s). CNS Neuroscience & Therapeutics published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Astrocyte-derived lactate aggravates brain injury of ischemic stroke in mice by promoting the formation of protein lactylation.Theranostics. 2024 Jul 8;14(11):4297-4317. doi: 10.7150/thno.96375. eCollection 2024. Theranostics. 2024. PMID: 39113798 Free PMC article.
-
Electroacupuncture suppresses NK cell infiltration and activation in the ischemic mouse brain through STAT3 inhibition.Brain Res Bull. 2024 Dec;219:111128. doi: 10.1016/j.brainresbull.2024.111128. Epub 2024 Nov 13. Brain Res Bull. 2024. PMID: 39542049
-
Electroacupuncture reduces cerebral ischemia-induced neuronal damage in the hippocampal CA1 region in rats by inhibiting HMGB1 and p-JNK overexpression.Int J Neurosci. 2025 Feb;135(2):132-139. doi: 10.1080/00207454.2023.2288541. Epub 2023 Nov 30. Int J Neurosci. 2025. PMID: 37999988
-
Electroacupuncture pretreatment attenuates cerebral ischemic injury through α7 nicotinic acetylcholine receptor-mediated inhibition of high-mobility group box 1 release in rats.J Neuroinflammation. 2012 Jan 26;9:24. doi: 10.1186/1742-2094-9-24. J Neuroinflammation. 2012. PMID: 22277256 Free PMC article.
-
[Electroacupuncture ameliorates ischemic injury in cerebral ischemia-reperfusion rats by regulating endogenous melatonin and inhibiting the activation of astrocytes].Zhen Ci Yan Jiu. 2022 Jan 25;47(1):39-45. doi: 10.13702/j.1000-0607.20210738. Zhen Ci Yan Jiu. 2022. PMID: 35128869 Chinese.
Cited by
-
Lactate Metabolism and Lactylation Modification: New Opportunities and Challenges in Cardiovascular Disease.MedComm (2020). 2025 Jul 1;6(7):e70269. doi: 10.1002/mco2.70269. eCollection 2025 Jul. MedComm (2020). 2025. PMID: 40599233 Free PMC article. Review.
References
-
- Virani S. S., Alonso A., Benjamin E. J., et al., “Heart Disease and Stroke Statistics‐2020 Update: A Report From the American Heart Association,” Circulation 141, no. 9 (2020): e139–e596. - PubMed
-
- Goyal M., Hill M. D., Saver J. L., and Fisher M., “Challenges and Opportunities of Endovascular Stroke Therapy,” Annals of Neurology 79, no. 1 (2016): 11–17. - PubMed
-
- Saver J. L., Goyal M., van der Lugt A., et al., “Time to Treatment With Endovascular Thrombectomy and Outcomes From Ischemic Stroke: A Meta‐Analysis,” JAMA 316, no. 12 (2016): 1279–1288. - PubMed
-
- Fransen P. S., Berkhemer O. A., Lingsma H. F., et al., “Time to Reperfusion and Treatment Effect for Acute Ischemic Stroke: A Randomized Clinical Trial,” JAMA Neurology 73, no. 2 (2016): 190–196. - PubMed
-
- Chia N. H., Leyden J. M., Newbury J., Jannes J., and Kleinig T. J., “Determining the Number of Ischemic Strokes Potentially Eligible for Endovascular Thrombectomy: A Population‐Based Study,” Stroke 47, no. 5 (2016): 1377–1380. - PubMed
MeSH terms
Substances
Grants and funding
- QNTD2023002/"Xinglin Scholars" Promotion Program of Chengdu University of Traditional Chinese Medicine
- 2024NSFSC0724/Natural Science Foundation of Sichuan Province
- ZYYCXTD-D-202003/Innovation Team and Talents Cultivation Program of National Administration of Traditional Chinese Medicine
- 82071334/National Natural Science Foundation of China
LinkOut - more resources
Full Text Sources

