The risk of failure of subcutaneous implantable cardioverter defibrillator therapy: from PRAETORIAN score to clinical practice
- PMID: 39834232
- PMCID: PMC11822678
- DOI: 10.1093/europace/euaf011
The risk of failure of subcutaneous implantable cardioverter defibrillator therapy: from PRAETORIAN score to clinical practice
Abstract
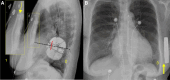
Aims: The subcutaneous implantable cardioverter defibrillator (S-ICD) is an alternative to traditional ICDs. The PRAETORIAN score, based on chest radiographs, has been validated to predict the probability of successful S-ICD defibrillation testing by assessing factors like fat thickness between the coil and sternum and generator placement. This study evaluated the correlation between the PRAETORIAN score and clinical characteristics, as well as implantation variables.
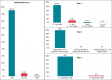
Methods and results: We retrospectively analysed data from 1253 patients who had undergone implantation of an S-ICD across 33 centres. The intermuscular positioning of the pulse generator was adopted in all patients. Post-implantation posterior-anterior and lateral chest radiographs were analysed to calculate the PRAETORIAN score. A total of 95.7% of patients had a PRAETORIAN score < 90, indicative of a low risk of conversion failure. Body mass index (BMI) was the only independent predictor of a score ≥ 90, and all patients with BMI < 25 kg/m2 (normal weight or underweight) had a score < 90. The intermuscular positioning technique resulted in optimal posterior placement of the device in all patients and significant sub-generator fat in only 3% of cases. A shock impedance value > 88 Ohm enabled to detect a PRAETORIAN score ≥ 90 with 98% (95% CI 97-99%) negative predictive value.
Conclusion: In contemporary practice, the PRAETORIAN score can be simplified. By adopting an intermuscular approach, two of the three steps of the score-evaluating the adequate posterior positioning of the generator and measuring the sub-generator fat-become superfluous, and impedance may serve as a reliable surrogate of sub-coil fat thickness. Furthermore, our data suggest that for non-obese patients, a favourable PRAETORIAN score is assured, making the score evaluation potentially unnecessary.
Clinical trial registration: URL: http://clinicaltrials.gov/ Identifier: NCT02275637.
Keywords: Conversion; Defibrillation test; Implantable defibrillator; Subcutaneous.
© The Author(s) 2025. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflict of interest: This was an independent study. No external funding was received for this project. M.Z. receives speaker’s fees and educational grant from Abbott, Boston Scientific, Biotronik, and Edwards Lifesciences. L.O. is consultant for Boston Scientific. P.F. received speaker’s fees and educational grants from Boston Scientific and research grants from Abbott. G.B. received speaker’s fee from Abbott, Biotronik, Boston Scientific, Medtronic, and Microport; R.R. received speaker’s fees from Abbot and Boston Scientific. M.L. and S.Va. are employees of Boston Scientific. The other authors report no conflicts.
Figures


References
-
- Knops RE, Olde Nordkamp LRA, Delnoy PHM, Boersma LVA, Kuschyk J, El-Chami MF et al. Subcutaneous or transvenous defibrillator therapy. N Engl J Med 2020;383:526–36. - PubMed
-
- Healey JS, Krahn AD, Bashir J, Amit G, Philippon F, McIntyre WF et al. Perioperative safety and early patient and device outcomes among subcutaneous versus transvenous implantable cardioverter defibrillator implantations: a randomized, multicenter trial. Ann Intern Med 2022;175:1658–65. - PubMed
-
- Heist EK, Belalcazar A, Stahl W, Brouwer TF, Knops RE. Determinants of subcutaneous implantable cardioverter-defibrillator efficacy: a computer modeling study. JACC Clin Electrophysiol 2017;3:405–14. - PubMed
-
- Quast ABE, Baalman SWE, Brouwer TF, Smeding L, Wilde AAM, Burke MC et al. A novel tool to evaluate the implant position and predict defibrillation success of the subcutaneous implantable cardioverter-defibrillator: the PRAETORIAN score. Heart Rhythm 2019;16:403–10. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

