Polarity and migration of cranial and cardiac neural crest cells: underlying molecular mechanisms and disease implications
- PMID: 39834387
- PMCID: PMC11743681
- DOI: 10.3389/fcell.2024.1457506
Polarity and migration of cranial and cardiac neural crest cells: underlying molecular mechanisms and disease implications
Abstract
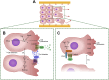
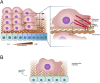
The Neural Crest cells are multipotent progenitor cells formed at the neural plate border that differentiate and give rise to a wide range of cell types and organs. Directional migration of NC cells and their correct positioning at target sites are essential during embryonic development, and defects in these processes results in congenital diseases. The NC migration begins with the epithelial-mesenchymal transition and extracellular matrix remodeling. The main cellular mechanisms that sustain this migration include contact inhibition of locomotion, co-attraction, chemotaxis and mechanical cues from the surrounding environment, all regulated by proteins that orchestrate cell polarity and motility. In this review we highlight the molecular mechanisms involved in neural crest cell migration and polarity, focusing on the role of small GTPases, Heterotrimeric G proteins and planar cell polarity complex. Here, we also discuss different congenital diseases caused by altered NC cell migration.
Keywords: cell migration; cell polarity; cell signaling; neural crest (NC); neural crest disorder.
Copyright © 2025 Salinas, Ruano-Rivadeneira, Leal, Caprile, Torrejón and Arriagada.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources

