Cu(OTf)2-catalyzed multicomponent reactions
- PMID: 39834894
- PMCID: PMC11744696
- DOI: 10.3762/bjoc.21.7
Cu(OTf)2-catalyzed multicomponent reactions
Abstract
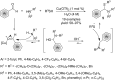
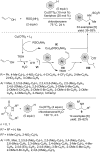
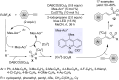
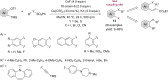
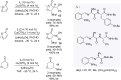
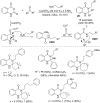
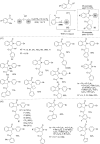
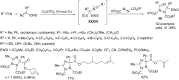
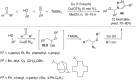
This review reports the achievements in copper(II) triflate-catalyzed processes concerning the multicomponent reactions, applied to the synthesis of acyclic and cyclic compounds. In particular, for the heteropolycyclic systems mechanistic insights were outlined as well as cycloaddition and aza-Diels-Alder reactions were included. These strategies have gained attention due to their highly atom- and step-economy, one-step multi-bond forming, mild reaction conditions, low cost and easy handling.
Keywords: cascade process; copper catalysis; heteropolycycles; multicomponent reactions; one-pot reaction.
Copyright © 2025, Colombo et al.
Figures


























References
-
- Tschan M J-L, Thomas C M, Strub H, Carpentier J-F. Adv Synth Catal. 2009;351(14-15):2496–2504. doi: 10.1002/adsc.200800750. - DOI
Publication types
LinkOut - more resources
Full Text Sources
