Unveiling the structural aspects of novel azo-dyes with promising anti-virulence activity against MRSA: a deep dive into the spectroscopy via integrated experimental and computational approaches
- PMID: 39835211
- PMCID: PMC11744518
- DOI: 10.1039/d4ra06367h
Unveiling the structural aspects of novel azo-dyes with promising anti-virulence activity against MRSA: a deep dive into the spectroscopy via integrated experimental and computational approaches
Abstract
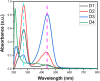
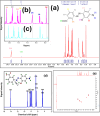
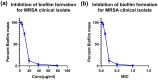
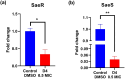
A novel series of azo dyes was successfully synthesized by combining amino benzoic acid and amino phenol on the same molecular framework via azo linkage. The structural elucidation of these dyes was carried out using various spectroscopic techniques, including UV-vis, FT-IR, NMR spectroscopy, and HRMS. Surprisingly, the aromatic proton in some dyes exhibited exchangeability in D2O, prompting a 2D NMR analysis to confirm this phenomenon. Furthermore, comprehensive density functional theory (DFT) calculations were conducted to unravel synthetic dyes' geometrical and electronic properties. Meanwhile, the reactivity of various sites was further investigated through Frontier Molecular Orbitals (FMOs) analysis and molecular electrostatic potential mapping. Besides, the experimental NMR spectra were interpreted by incorporating theoretically computed NMR spectrum and reduced density gradient (RDG) function. These computations revealed a pronounced intramolecular hydrogen bond through O-H⋯N interaction that significantly influenced the proton chemical shift. The dyes were assessed for their antimicrobial activities using agar diffusion, micro broth dilution, and biofilm inhibition assays. Interestingly, one of the synthetic dyes showed promising antibacterial effects against S. aureus (ATCC-6538) as well as against a multidrug-resistant MRSA clinical isolate with a MIC (minimum inhibitory concentration) of 78.12 μg mL-1. Moreover, that dye inhibited biofilm formation of the strong biofilm former clinical MRSA isolate with a concentration as low as 0.25 MIC (19.53 μg mL-1). Indeed, our qPCR data suggest that inhibiting the SaeS/SaeR system is another potential mechanism by which D4 exerts its antibacterial and anti-virulence effects. Altogether, this shows these synthetic azo dyes' promising antibacterial and anti-virulence activities concerning MRSA clinical infections.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




Similar articles
-
Experimental and Computational Study of Novel Pyrazole Azo Dyes as Colored Materials for Light Color Paints.Materials (Basel). 2022 Aug 11;15(16):5507. doi: 10.3390/ma15165507. Materials (Basel). 2022. PMID: 36013644 Free PMC article.
-
Novel edaravone-based azo dyes: efficient synthesis, characterization, antibacterial activity, DFT calculations and comprehensive investigation of the solvent effect on the absorption spectra.RSC Adv. 2020 Sep 29;10(59):35729-35739. doi: 10.1039/d0ra06934e. eCollection 2020 Sep 28. RSC Adv. 2020. PMID: 35517118 Free PMC article.
-
Exploring Pyrimidine-Based azo Dyes: Vibrational spectroscopic Assignments, TD-DFT Investigation, chemical Reactivity, HOMO-LUMO, ELF, LOL and NCI-RDG analysis.Spectrochim Acta A Mol Biomol Spectrosc. 2024 May 15;313:124093. doi: 10.1016/j.saa.2024.124093. Epub 2024 Feb 28. Spectrochim Acta A Mol Biomol Spectrosc. 2024. PMID: 38428162
-
Synthesis, structural characterization, antimicrobial activities and theoretical investigations of some 4-(4-aminophenylsulfonyl) phenylimino) methyl)-4-(aryldiazenyl) phenol.Spectrochim Acta A Mol Biomol Spectrosc. 2016 Nov 5;168:190-198. doi: 10.1016/j.saa.2016.06.007. Epub 2016 Jun 6. Spectrochim Acta A Mol Biomol Spectrosc. 2016. PMID: 27294547
-
Microwave assisted synthesis, experimental and theoretical characterization and antibacterial activity screening of novel azomethine compounds containing thiophene and aminophenol functionality.Spectrochim Acta A Mol Biomol Spectrosc. 2020 Dec 15;243:118761. doi: 10.1016/j.saa.2020.118761. Epub 2020 Aug 3. Spectrochim Acta A Mol Biomol Spectrosc. 2020. PMID: 32854082
References
-
- Guerroudj A. R. Mughal E. U. Naeem N. Sadiq A. Al-Fahemi J. H. Asghar B. H. Boukabcha N. Chouaih A. Ahmed S. A. Spectrochim. Acta, Part A. 2024:124093. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

