Nerve repair with polylactic acid and inosine treatment enhance regeneration and improve functional recovery after sciatic nerve transection
- PMID: 39835292
- PMCID: PMC11743644
- DOI: 10.3389/fncel.2024.1525024
Nerve repair with polylactic acid and inosine treatment enhance regeneration and improve functional recovery after sciatic nerve transection
Abstract
Background: Following transection, nerve repair using the polylactic acid (PLA) conduit is an effective option. In addition, inosine treatment has shown potential to promote nerve regeneration. Therefore, this study aimed to investigate the regenerative potential of inosine after nerve transection and polylactic acid conduit repair.
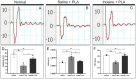
Methods: C57/Black6 mice were subjected to sciatic nerve transection, repair with PLA conduit, and intraperitoneal injection of saline or inosine 1 h after injury and daily for 1 week. To assess motor and sensory recovery, functional tests were performed before and weekly up to 8 weeks after injury. Following, to evaluate the promotion of regeneration and myelination, electroneuromyography, morphometric analysis and immunohistochemistry were then performed.
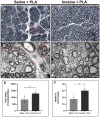
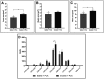
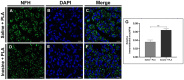
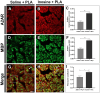
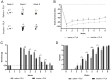
Results: Our results showed that the inosine group had a greater number of myelinated nerve fibers (1,293 ± 85.49 vs. 817 ± 89.2), an increase in neurofilament high chain (NFH) and myelin basic protein (MBP) immunolabeling and a greater number of fibers within the ideal g-ratio (453.8 ± 45.24 vs. 336.6 ± 37.01). In addition, the inosine group presented a greater adenosine A2 receptor (A2AR) immunolabeling area. This resulted in greater compound muscle action potential amplitude and nerve conduction velocity, leading to preservation of muscle and neuromuscular junction integrity, and consequently, the recovery of motor and sensory function.
Conclusion: Our findings suggest that inosine may enhance regeneration and improve both motor and sensory function recovery after nerve transection when repaired with a poly-lactic acid conduit. This advances the understanding of biomaterials and molecular treatments.
Keywords: A2A receptor; inosine; nerve regeneration; nerve repair; sciatic nerve transection.
Copyright © 2025 Cardoso, Maria, Pestana, Cardoso, Ramalho, Heringer, Taboada, Martinez and Almeida. Cardoso, Maria, Pestana, Cardoso, Ramalho, Heringer, Taboada, Martinez and Almeida.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
LinkOut - more resources
Full Text Sources
Miscellaneous

