Pimobendan oral solution is bioequivalent to pimobendan chewable tablets in beagle dogs
- PMID: 39835518
- PMCID: PMC11747864
- DOI: 10.1111/jvim.17248
Pimobendan oral solution is bioequivalent to pimobendan chewable tablets in beagle dogs
Abstract
Background: Myxomatous mitral valve disease (MMVD) is frequently diagnosed in small breed dogs. Pimobendan oral solution has been developed to improve dosing accuracy in small and toy breed dogs.
Hypothesis/objectives: Demonstrate bioequivalence of pimobendan oral solution with pimobendan chewable tablets using a pharmacokinetic and a pharmacodynamic study in healthy purpose bred dogs.
Animals: In the pharmacokinetic study, 24 beagle dogs were dosed in a 4-period crossover design. In the pharmacodynamic study, 4 mongrel and 2 beagle dogs implanted with telemetry probes were included in a 2-way crossover design.
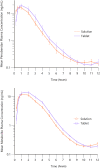
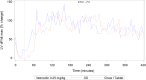
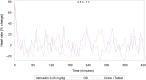
Methods: Both studies were designed as prospective, randomized crossover trials. Dogs were given single doses of 5 mg/dog of either formulation followed by serial blood sampling for determination of pimobendan and O-desmethyl-pimobendan (ODMP; main metabolite). Because of high variability in the pharmacokinetics, the reference scaled average bioequivalence (RSABE) method was applied. For the pharmacodynamic study, animals were dosed with 0.25 mg/kg of either formulation. Baseline corrected left ventricular maximal pressure (LVdP/dtmax) and heart rate were recorded continuously and compared with a predefined bioequivalence threshold.
Results: Pimobendan was verified as a high variability drug. Based on the RSABE method, both formulations were bioequivalent. Pharmacodynamic results supported bioequivalence.
Conclusions and clinical importance: The novel oral solution of pimobendan was found to be bioequivalent, both applying the Food and Drug Administration (FDA) supported RSABE method and based on pharmacodynamic data. Thus, the novel liquid formulation can be used to facilitate accurate dosing of small and toy breed dogs.
Keywords: RSABE; bioequivalence; dog; pharmacodynamic; pharmacokinetics; pharmacology.
© 2025 The Author(s). Journal of Veterinary Internal Medicine published by Wiley Periodicals LLC on behalf of American College of Veterinary Internal Medicine.
Conflict of interest statement
Dr Olaf Kuhlmann and Michael Markert are both employees of Boehringer Ingelheim, the company which has developed and marketed the originator product as well as the novel oral solution under the brand name Vetmedin (pimobendan oral solution) 1.5 mg/mL. The study was fully funded by Boehringer Ingelheim, Germany.
Figures
References
-
- Häggström J, Boswood A, O'Grady M, et al. Effect of pimobendan or benazepril hydrochloride on survival times in dogs with congestive heart failure caused by naturally occurring myxomatous mitral valve disease: the QUEST study. J Vet Intern Med. 2008;22(5):1124‐1135. - PubMed
-
- Boswood A, Gordon SG, Häggström J, et al. Longitudinal analysis of quality of life, clinical, radiographic, echocardiographic, and laboratory variables in dogs with preclinical myxomatous mitral valve disease receiving Pimobendan or placebo: the EPIC study. J Vet Intern Med. 2018;32(1):72‐85. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

