Annexin A8 deficiency delays atherosclerosis progression
- PMID: 39835780
- PMCID: PMC11748212
- DOI: 10.1002/ctm2.70176
Annexin A8 deficiency delays atherosclerosis progression
Abstract
Background: Atherosclerosis is a chronic inflammatory disease characterized by the accumulation of lipids and leukocytes within the arterial wall. By studying the aortic transcriptome of atherosclerosis-prone apolipoprotein E (ApoE-/-) mice, we aimed to identify novel players in the progression of atherosclerosis.
Methods: RNA-Seq analysis was performed on aortas from ApoE-/- and wild-type mice. AnxA8 expression was assessed in human and mice atherosclerotic tissue and healthy aorta. ApoE-/- mice lacking systemic AnxA8 (ApoE-/-AnxA8-/-) were generated to assess the effect of AnxA8 deficiency on atherosclerosis. Bone marrow transplantation (BMT) was also performed to generate ApoE-/- lacking AnxA8 specifically in bone marrow-derived cells. Endothelial-specific AnxA8 silencing in vivo was performed in ApoE-/- mice. The functional role of AnxA8 was analysed in cultured murine cells.
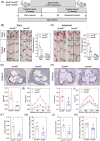
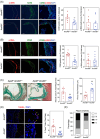
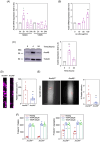
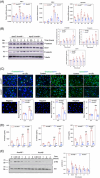
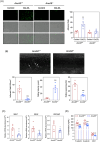
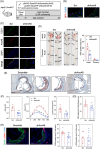
Results: RNA-Seq unveiled AnxA8 as one of the most significantly upregulated genes in atherosclerotic aortas of ApoE-/- compared to wild-type mice. Moreover, AnxA8 was upregulated in human atherosclerotic plaques. Germline deletion of AnxA8 decreased the atherosclerotic burden, the size and volume of atherosclerotic plaques in the aortic root. Plaques of ApoE-/-AnxA8-/- were characterized by lower lipid and inflammatory content, smaller necrotic core, thicker fibrous cap and less apoptosis compared with those in ApoE-/-AnxA8+/+. BMT showed that hematopoietic AnxA8 deficiency had no effect on atherosclerotic progression. Oxidized low-density lipoprotein (ox-LDL) increased AnxA8 expression in murine aortic endothelial cells (MAECs). In vitro experiments revealed that AnxA8 deficiency in MAECs suppressed P/E-selectin and CD31 expression and secretion induced by ox-LDL with a concomitant reduction in platelet and leukocyte adhesion. Intravital microscopy confirmed the reduction in leukocyte and platelet adhesion in ApoE-/-AnxA8-/- mice. Finally, endothelial-specific silencing of AnxA8 decreased atherosclerosis progression.
Conclusion: Our findings demonstrate that AnxA8 promotes the progression of atherosclerosis by modulating endothelial-leukocyte interactions. Interventions capable of reducing AnxA8 expression in endothelial cells may delay atherosclerotic plaque progression.
Key points: This study shows that AnxA8 is upregulated in aorta of atheroprone mice and in human atherosclerotic plaques. Germline AnxA8 deficiency reduces platelet and leukocyte recruitment to activated endothelium as well as atherosclerotic burden, plaque size, and macrophage accumulation in mice. AnxA8 regulates oxLDL-induced adhesion molecules expression in aortic endothelial cells. Our data strongly suggest that AnxA8 promotes disease progression through regulation of adhesion and influx of immune cells to the intima. Endothelial specific silencing of AnxA8 reduced atherosclerosis progression. Therapeutic interventions to reduce AnxA8 expression may delay atherosclerosis progression.
Keywords: AnxA8; atherosclerosis; inflammation.
© 2025 The Author(s). Clinical and Translational Medicine published by John Wiley & Sons Australia, Ltd on behalf of Shanghai Institute of Clinical Bioinformatics.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous
