Paradoxical dominant negative activity of an immunodeficiency-associated activating PIK3R1 variant
- PMID: 39835783
- PMCID: PMC11750134
- DOI: 10.7554/eLife.94420
Paradoxical dominant negative activity of an immunodeficiency-associated activating PIK3R1 variant
Abstract
PIK3R1 encodes three regulatory subunits of class IA phosphoinositide 3-kinase (PI3K), each associating with any of three catalytic subunits, namely p110α, p110β, or p110δ. Constitutional PIK3R1 mutations cause diseases with a genotype-phenotype relationship not yet fully explained: heterozygous loss-of-function mutations cause SHORT syndrome, featuring insulin resistance and short stature attributed to reduced p110α function, while heterozygous activating mutations cause immunodeficiency, attributed to p110δ activation and known as APDS2. Surprisingly, APDS2 patients do not show features of p110α hyperactivation, but do commonly have SHORT syndrome-like features, suggesting p110α hypofunction. We sought to investigate this. In dermal fibroblasts from an APDS2 patient, we found no increased PI3K signalling, with p110δ expression markedly reduced. In preadipocytes, the APDS2 variant was potently dominant negative, associating with Irs1 and Irs2 but failing to heterodimerise with p110α. This attenuation of p110α signalling by a p110δ-activating PIK3R1 variant potentially explains co-incidence of gain-of-function and loss-of-function PIK3R1 phenotypes.
Keywords: APDS2; PIK3R1; SHORT syndrome; genetics; genomics; human; immunodeficiency; immunology; inflammation; insulin resistance; mouse; phosphoinositide 3-kinase.
Conflict of interest statement
PT, RK, OP, HS, GB, RW No competing interests declared, RS Consulting for Novartis on clinical aspects of PIK3CA-related overgrowth, and for Alnylam, Amryt and AstraZeneca on clinical aspects of monogenic insulin resistance and lipodystrophy
Figures













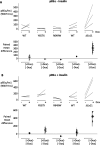
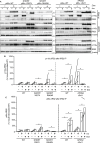


Update of
-
Paradoxical dominant negative activity of an immunodeficiency-associated activating PIK3R1 variant.bioRxiv [Preprint]. 2024 Nov 5:2023.11.02.565250. doi: 10.1101/2023.11.02.565250. bioRxiv. 2024. Update in: Elife. 2025 Jan 21;13:RP94420. doi: 10.7554/eLife.94420. PMID: 38077044 Free PMC article. Updated. Preprint.
References
-
- Angulo I, Vadas O, Garçon F, Banham-Hall E, Plagnol V, Leahy TR, Baxendale H, Coulter T, Curtis J, Wu C, Blake-Palmer K, Perisic O, Smyth D, Maes M, Fiddler C, Juss J, Cilliers D, Markelj G, Chandra A, Farmer G, Kielkowska A, Clark J, Kracker S, Debré M, Picard C, Pellier I, Jabado N, Morris JA, Barcenas-Morales G, Fischer A, Stephens L, Hawkins P, Barrett JC, Abinun M, Clatworthy M, Durandy A, Doffinger R, Chilvers ER, Cant AJ, Kumararatne D, Okkenhaug K, Williams RL, Condliffe A, Nejentsev S. Phosphoinositide 3-kinase δ gene mutation predisposes to respiratory infection and airway damage. Science. 2013;342:866–871. doi: 10.1126/science.1243292. - DOI - PMC - PubMed
-
- Avila M, Dyment DA, Sagen JV, St-Onge J, Moog U, Chung BHY, Mo S, Mansour S, Albanese A, Garcia S, Martin DO, Lopez AA, Claudi T, König R, White SM, Sawyer SL, Bernstein JA, Slattery L, Jobling RK, Yoon G, Curry CJ, Merrer ML, Luyer BL, Héron D, Mathieu-Dramard M, Bitoun P, Odent S, Amiel J, Kuentz P, Thevenon J, Laville M, Reznik Y, Fagour C, Nunes ML, Delesalle D, Manouvrier S, Lascols O, Huet F, Binquet C, Faivre L, Rivière JB, Vigouroux C, Njølstad PR, Innes AM, Thauvin-Robinet C. Clinical reappraisal of SHORT syndrome with PIK3R1 mutations: toward recommendation for molecular testing and management. Clinical Genetics. 2016;89:501–506. doi: 10.1111/cge.12688. - DOI - PubMed
-
- Brachmann SM, Ueki K, Engelman JA, Kahn RC, Cantley LC. Phosphoinositide 3-kinase catalytic subunit deletion and regulatory subunit deletion have opposite effects on insulin sensitivity in mice. Molecular and Cellular Biology. 2005;25:1596–1607. doi: 10.1128/MCB.25.5.1596-1607.2005. - DOI - PMC - PubMed
-
- Bravo García-Morato M, García-Miñaúr S, Molina Garicano J, Santos Simarro F, Del Pino Molina L, López-Granados E, Ferreira Cerdán A, Rodríguez Pena R. Mutations in PIK3R1 can lead to APDS2, SHORT syndrome or a combination of the two. Clinical Immunology. 2017;179:77–80. doi: 10.1016/j.clim.2017.03.004. - DOI - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

