Bone-Induced HER2 Promotes Secondary Metastasis in HR+/HER2- Breast Cancer
- PMID: 39835789
- PMCID: PMC11964846
- DOI: 10.1158/2159-8290.CD-23-0543
Bone-Induced HER2 Promotes Secondary Metastasis in HR+/HER2- Breast Cancer
Abstract
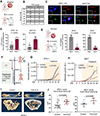
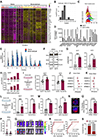
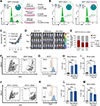
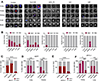
Given the urgent need for alternative strategies to block metastasis progression, we demonstrate that blocking HER2-mediated secondary metastasis improves clinical outcome and establish HER2 as a biomarker for bone metastasis in patients with initial HR+/HER2- breast cancer, which represents ∼70% of all cases.
©2025 American Association for Cancer Research.
Conflict of interest statement
The authors declare no potential conflicts of interest.
Figures



References
-
- Paterson AHG, Anderson SJ, Lembersky BC, Fehrenbacher L, Falkson CI, King KM, et al. Oral clodronate for adjuvant treatment of operable breast cancer (National Surgical Adjuvant Breast and Bowel Project protocol B-34): A multicentre, placebo-controlled, randomised trial. Lancet Oncol 2012;13:734–42. - PMC - PubMed
-
- Gnant M, Mlineritsch B, Stoeger H, Luschin-Ebengreuth G, Knauer M, Moik M, et al. Zoledronic acid combined with adjuvant endocrine therapy of tamoxifen versus anastrozol plus ovarian function suppression in premenopausal early breast cancer: Final analysis of the Austrian Breast and Colorectal Cancer Study Group Trial 12. Annals of Oncology 2015;26:313–20. - PubMed
-
- Von Minckwitz G, Möbus V, Schneeweiss A, Huober J, Thomssen C, Untch M, et al. German adjuvant intergroup node-positive study: A phase III trial to compare oral ibandronate versus observation in patients with high-risk early breast cancer. Journal of Clinical Oncology 2013;31:3531–9. - PubMed
-
- Coleman R, Powles T, Paterson A, Gnant M, Anderson S, Diel I, et al. Adjuvant bisphosphonate treatment in early breast cancer: meta-analyses of individual patient data from randomised trials. Lancet. Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Open Access article distributed under the terms of CC BY; 2015;386:1353–61. - PubMed
-
- Coleman R, De Boer R, Eidtmann H, Llombart A, Davidson N, Neven P, et al. Zoledronic acid (zoledronate) for postmenopausal women with early breast cancer receiving adjuvant letrozole (ZO-FAST study): Final 60-month results. Annals of Oncology 2013;24:398–405. - PubMed
MeSH terms
Substances
Grants and funding
- S10 OD018033/OD/NIH HHS/United States
- S10 OD025240/OD/NIH HHS/United States
- R01 CA267696/CA/NCI NIH HHS/United States
- R01CA183878/National Cancer Institute (NCI)
- S10 OD026880/OD/NIH HHS/United States
- W81XWH-21-1-0790/U.S. Department of Defense (DOD)
- 23-20-26-BADO/Breast Cancer Research Foundation (BCRF)
- R01 CA271346/CA/NCI NIH HHS/United States
- S10 RR024574/RR/NCRR NIH HHS/United States
- U54CA267776/Common Fund (NIH Common Fund)
- P30 CA125123/CA/NCI NIH HHS/United States
- U01 CA253553/CA/NCI NIH HHS/United States
- CPRIT-RP180672/Cancer Prevention and Research Institute of Texas (CPRIT)
- S10 OD023469/OD/NIH HHS/United States
- U54 CA267776/CA/NCI NIH HHS/United States
- R00 CA263033/CA/NCI NIH HHS/United States
- R01 CA251950/CA/NCI NIH HHS/United States
- S10 OD030463/OD/NIH HHS/United States
- P30CA196521/National Institutes of Health (NIH)
- R01 CA183878/CA/NCI NIH HHS/United States
- K99CA263033-01A1/National Cancer Institute (NCI)
- Breast Cancer Alliance (BCA)
- R01 CA237264/CA/NCI NIH HHS/United States
- P30 CA196521/CA/NCI NIH HHS/United States
- K99 CA263033/CA/NCI NIH HHS/United States
- BC201371P1/U.S. Department of Defense (DOD)
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

