Nav1.8, an analgesic target for nonpsychotomimetic phytocannabinoids
- PMID: 39835903
- PMCID: PMC11789019
- DOI: 10.1073/pnas.2416886122
Nav1.8, an analgesic target for nonpsychotomimetic phytocannabinoids
Abstract
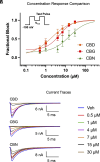
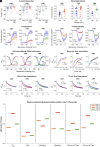
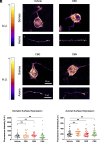
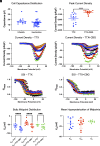
Pain impacts billions of people worldwide, but treatment options are limited and have a spectrum of adverse effects. The search for safe and nonaddictive pain treatments has led to a focus on key mediators of nociceptor excitability. Voltage-gated sodium (Nav) channels in the peripheral nervous system-Nav1.7, Nav1.8, and Nav1.9-play crucial roles in pain signaling. Among these, Nav1.8 has shown promise due to its rapid recovery from inactivation and role in repetitive firing, with recent clinical studies providing proof-of-principal that block of Nav1.8 can reduce pain in humans. We report here that three nonpsychotomimetic cannabinoids-cannabidiol (CBD), cannabigerol (CBG), and cannabinol (CBN)-effectively inhibit Nav1.8, suggesting their potential as analgesic compounds. In particular, CBG shows significant promise due to its ability to effectively inhibit excitability of peripheral sensory neurons. These findings highlight the therapeutic potential of cannabinoids, particularly CBG, as agents that may attenuate pain via block of Nav1.8, warranting further in vivo studies.
Keywords: cannabidiol; cannabigerol; cannabinol; sensory neurons; voltage-gated sodium channel.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures

Similar articles
-
A novel Nav1.7, Nav1.8, and Nav1.9 blocker, ANP-230, has broad analgesic efficacy in preclinical pain models with favorable safety margins.Biochem Biophys Res Commun. 2025 Sep 1;777:152197. doi: 10.1016/j.bbrc.2025.152197. Epub 2025 Jun 16. Biochem Biophys Res Commun. 2025. PMID: 40633498
-
Modulation of human dorsal root ganglion neuron firing by the Nav1.8 inhibitor suzetrigine.Proc Natl Acad Sci U S A. 2025 Jun 3;122(22):e2503570122. doi: 10.1073/pnas.2503570122. Epub 2025 May 27. Proc Natl Acad Sci U S A. 2025. PMID: 40424150
-
Conservation of alternative splicing in sodium channels reveals evolutionary focus on release from inactivation and structural insights into gating.J Physiol. 2017 Aug 15;595(16):5671-5685. doi: 10.1113/JP274693. Epub 2017 Jul 18. J Physiol. 2017. PMID: 28621020 Free PMC article.
-
Ion channels and G protein-coupled receptors: Cannabidiol actions on disorders of excitability and synaptic excitatory-inhibitory ratio.Mol Pharmacol. 2025 Mar;107(3):100017. doi: 10.1016/j.molpha.2025.100017. Epub 2025 Feb 7. Mol Pharmacol. 2025. PMID: 40048808 Review.
-
Sensory neuron sodium channels as pain targets; from cocaine to Journavx (VX-548, suzetrigine).J Gen Physiol. 2025 Jul 7;157(4):e202513778. doi: 10.1085/jgp.202513778. Epub 2025 Apr 28. J Gen Physiol. 2025. PMID: 40294084 Review.
Cited by
-
Corneal Sensory Receptors and Pharmacological Therapies to Modulate Ocular Pain.Int J Mol Sci. 2025 May 13;26(10):4663. doi: 10.3390/ijms26104663. Int J Mol Sci. 2025. PMID: 40429806 Free PMC article. Review.
-
Targeting sodium channels - challenges for acute pain and the leap to chronic pain.Nat Rev Neurol. 2025 Aug 15. doi: 10.1038/s41582-025-01127-1. Online ahead of print. Nat Rev Neurol. 2025. PMID: 40817289 Review.
-
High-throughput multiplex voltage-clamp/current-clamp evaluation of acutely isolated neurons.Nat Protoc. 2025 Jun 13. doi: 10.1038/s41596-025-01194-0. Online ahead of print. Nat Protoc. 2025. PMID: 40514421 Review.
-
In vitro inhibition of voltage-dependent sodium currents by the antifungal drug amorolfine.J Biol Chem. 2025 Apr;301(4):108407. doi: 10.1016/j.jbc.2025.108407. Epub 2025 Mar 14. J Biol Chem. 2025. PMID: 40090585 Free PMC article.
References
-
- Dib-Hajj S. D., Cummins T. R., Black J. A., Waxman S. G., Sodium channels in normal and pathological pain. Annu. Rev. Neurosci. 33, 325–347 (2010). - PubMed
-
- Waxman S. G., Targeting a peripheral sodium channel to treat pain. N. Engl. J. Med. 389, 466–469 (2023). - PubMed
-
- Akopian A. N., Sivilotti L., Wood J. N., A tetrodotoxin-resistant voltage-gated sodium channel expressed by sensory neurons. Nature 379, 257–262 (1996). - PubMed
-
- Sangameswaran L., et al. , Structure and function of a novel voltage-gated, tetrodotoxin-resistant sodium channel specific to sensory neurons. J. Biol. Chem. 271, 5953–5956 (1996). - PubMed
-
- Renganathan M., Cummins T. R., Waxman S. G., Contribution of Na(v)1.8 sodium channels to action potential electrogenesis in DRG neurons. J. Neurophysiol. 86, 629–640 (2001). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

