Secondhand vape exposure regulation of CFTR and immune function in cystic fibrosis
- PMID: 39836014
- PMCID: PMC12164564
- DOI: 10.1152/ajplung.00328.2024
Secondhand vape exposure regulation of CFTR and immune function in cystic fibrosis
Abstract
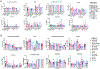
Secondhand smoke exposure (SHSe) is a public health threat for people with cystic fibrosis (CF) and other lung diseases. Primary smoking reduces CF transmembrane conductance regulator (CFTR) channel function, the causative defect in CF. We reported that SHSe worsens respiratory and nutritional outcomes in CF by disrupting immune responses and metabolic signaling. Recently, electronic cigarette (e-cigs) usage by caregivers and peers has increased rapidly, causing new secondhand e-cig vape exposures. Primary vaping is associated with immunologic deficits in healthy people, but it is unknown whether e-cigs similarly impacts CF immune function or how it differs from SHSe. Human CF and non-CF blood monocyte-derived macrophages (MDMs) and bronchial epithelial cells (HBECs) were exposed to flavored and unflavored e-cigs. The effect of e-cigs on CFTR expression and function, bacterial killing, cytokine signaling, lipid mediators, and metabolism was measured during treatment with CFTR modulators. E-cigs decreased CFTR expression and function in CF and non-CF MDMs and negated CFTR functional restoration by elexacaftor/tezacaftor/ivacaftor (ETI). E-cigs also negated the restoration of anti-inflammatory PGD2 expression in CF MDMs treated with ETI compared with controls. Flavored but not unflavored e-cigs increased proinflammatory cytokine expression in CF MDMs and e-cigs promoted glycolytic metabolism. E-cigs did not impact bacterial killing. Overall, HBECs were less impacted by e-cigs compared with MDMs. E-cigs reduced macrophage CFTR expression and hindered functional CFTR restoration by CFTR modulators, promoting a glycolytic, proinflammatory state. E-cigs are an emerging public health threat that may limit the efficacy of CFTR modulators in people with CF.NEW & NOTEWORTHY New research reveals that e-cigarettes pose a serious health risk for individuals with cystic fibrosis (CF). Exposure to electronic cigarette (e-cig) vapors decreases CF transmembrane conductance regulator (CFTR) function and undermines the effectiveness of CFTR modulators, potentially worsening inflammation and metabolic responses. This highlights an urgent need for awareness around e-cig use, especially among caregivers and peers of those with CF. E-cigarettes may further complicate the management of this chronic lung disease.
Keywords: cystic fibrosis; inflammation; modulators; omics; vaping.
Copyright © 2025 The Authors.
Conflict of interest statement
Figures
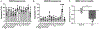
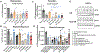
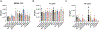

References
-
- Tyrrell J, Ghosh A, Manzo ND, Randell SH, and Tarran R. Evaluation of chronic cigarette smoke exposure in human bronchial epithelial cultures. J Appl Toxicol 43: 862–873, 2023. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

