CaMKIIγ advances chronic intermittent hypoxia-induced cardiomyocyte apoptosis via HIF-1 signaling pathway
- PMID: 39836257
- PMCID: PMC11750943
- DOI: 10.1007/s11325-024-03225-8
CaMKIIγ advances chronic intermittent hypoxia-induced cardiomyocyte apoptosis via HIF-1 signaling pathway
Abstract
Background: Our previous study have demonstrated chronic intermittent hypoxia (CIH) induced cardiomyocyte apoptosis and cardiac dysfunction. However, the molecular mechanisms are complicated and varied. In this study, we first investigated the CaMKIIγ expression and signaling pathway in the pathogenesis of cardiomyocyte apoptosis after CIH.
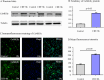
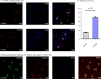
Methods: Rats were separated into CIH and Normoxia groups, and H9c2 cells were divided into Control and CIH + 8 h groups. Rat body weight (BW) was markedly gained from two to six weeks. Furthermore, CIH decreased cardiac dysfunction, damaged cellular structure, induced myocardial fibrosis, and promoted cardiomyocyte apoptosis by HE, masson, sirius-red, and TUNEL staining. Western blot, immunohistochemical, immunofluorescence, double immunofluorescence staining were performed to investigate CaMKIIγ, Bcl-2, Bax, Caspase 3, HIF-1 protein expression.
Results: Heart weight (HW) and HW/BW ratio in CIH group was markedly gained compared with the Normoxia group. CaMKIIγ expression was notably increased after CIH, and mainly expressed in the cytoplasm in vivo and vitro. The results of HIF-1 expression have the same trend of CaMKIIγ expression and cardiomyocyte apoptosis. In addition, the co-localizations of CaMKIIγ with Caspase 3, and CaMKIIγ with HIF-1 were observed by double immunofluorescence staining.
Conclusions: These results indicated increased CaMKIIγ expression advances CIH-induced cardiomyocyte apoptosis via HIF-1 signaling pathway, which afford a new insight and provide a potential therapy for OSA patients.
Keywords: Apoptosis; CaMKIIγ; Cardiomyocyte; Chronic intermittent hypoxia; H9c2 cells; HIF-1.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethical approval: The protocol of this study was approved by the Jiangsu Province Animal Care ethics committee and performed in accordance with the Animal Management Rule of the People’s Republic of China and the Care and Use of the Laboratory Animals Guide of the Nantong University. Conflict of interest: All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Figures





Similar articles
-
Cx43 overexpression reduce the incidence of obstructive sleep apnea associated atrial fibrillation via the CaMKⅡγ/HIF-1 axis.Biochem Biophys Res Commun. 2023 Jun 4;659:62-71. doi: 10.1016/j.bbrc.2023.03.084. Epub 2023 Mar 31. Biochem Biophys Res Commun. 2023. PMID: 37037067
-
Overexpression of filamin c in chronic intermittent hypoxia-induced cardiomyocyte apoptosis is a potential cardioprotective target for obstructive sleep apnea.Sleep Breath. 2019 Jun;23(2):493-502. doi: 10.1007/s11325-018-1712-9. Epub 2018 Sep 7. Sleep Breath. 2019. PMID: 30194514
-
Chronic intermittent hypoxia aggravates cardiomyocyte apoptosis in rat ovariectomized model.Chin Med J (Engl). 2012 Sep;125(17):3087-92. Chin Med J (Engl). 2012. PMID: 22932186
-
[MicroRNA-1249 regulates the apoptosis of myocardial cells in rats with chronic intermittent hypoxia by autophagy].Zhonghua Yi Xue Za Zhi. 2018 Sep 25;98(36):2937-2941. doi: 10.3760/cma.j.issn.0376-2491.2018.36.013. Zhonghua Yi Xue Za Zhi. 2018. PMID: 30293354 Chinese.
-
[Effects of chronic intermittent hypoxia on regulation of miRNA-214 in myocardial apoptosis in rats].Zhonghua Yi Xue Za Zhi. 2015 Apr 28;95(16):1214-7. Zhonghua Yi Xue Za Zhi. 2015. PMID: 26081503 Chinese.
References
-
- Lyons MM, Bhatt NY, Pack AI, Magalang UJ (2020) Global burden of sleep-disordered breathing and its implications. Respirology 25(7):690–702. 10.1111/resp.13838 - PubMed
-
- Benjafield AV, Ayas NT, Eastwood PR, Heinzer R, Ip MSM, Morrell MJ, Nunez CM, Patel SR, Penzel T, Pepin JL, Peppard PE, Sinha S, Tufik S, Valentine K, Malhotra A (2019) Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respiratory Med 7(8):687–698. 10.1016/S2213-2600(19)30198-5 - PMC - PubMed
-
- Srichan S, Phannajit J, Tungsanga S, Jaimchariyatam N (2023) The NH-OSA score in prediction of clinically significant obstructive sleep apnea among the Thai population: derivation and validation studies. Sleep Breath = Schlaf Atmung 27(3):913–921. 10.1007/s11325-022-02642-x - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

