Aging and tumors: a dynamic interaction
- PMID: 39836268
- PMCID: PMC11751271
- DOI: 10.1007/s12672-025-01808-9
Aging and tumors: a dynamic interaction
Abstract
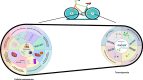
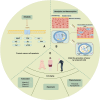
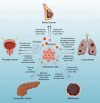
Aging is an inevitable physiological process in organisms, and the development of tumors is closely associated with cellular senescence. This article initially examines the role of cellular senescence in tumorigenesis, emphasizing the correlation between telomere length-a marker of cellular senescence-and tumor risk. Concurrently, the study explores the expression levels of senescence-associated markers, such as p16, p53, and mTOR, in the context of tumor development. Additionally, the study investigates the impact of tumors on cellular and organismal senescence, including the effects on immune system function and metabolic processes. Ultimately, the discussion explores the potential application of anti-aging strategies in tumor therapy and considers the possibility of utilizing senescence mechanisms as a novel therapeutic approach for tumors. This research provides novel insights into the complex interplay between senescence and tumor development, suggesting potential strategies for future preventative measures and therapeutic interventions.
Keywords: Aging; Anti-aging treatment; Cellular senescence; Telomere length; Tumor.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures
Similar articles
-
Senescence research from historical theory to future clinical application.Geriatr Gerontol Int. 2021 Feb;21(2):125-130. doi: 10.1111/ggi.14121. Epub 2020 Dec 28. Geriatr Gerontol Int. 2021. PMID: 33372374 Review.
-
Cellular Senescence in Diabetes Mellitus: Distinct Senotherapeutic Strategies for Adipose Tissue and Pancreatic β Cells.Front Endocrinol (Lausanne). 2022 Mar 31;13:869414. doi: 10.3389/fendo.2022.869414. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35432205 Free PMC article. Review.
-
Chronic stress exposure and daily stress appraisals relate to biological aging marker p16INK4a.Psychoneuroendocrinology. 2019 Apr;102:139-148. doi: 10.1016/j.psyneuen.2018.12.006. Epub 2018 Dec 7. Psychoneuroendocrinology. 2019. PMID: 30557761 Free PMC article.
-
Cancer, aging and cellular senescence.In Vivo. 2000 Jan-Feb;14(1):183-8. In Vivo. 2000. PMID: 10757076 Review.
-
Quantitative proteomic profiling of tumor cell response to telomere dysfunction using isotope-coded protein labeling (ICPL) reveals interaction network of candidate senescence markers.J Proteomics. 2013 Oct 8;91:515-35. doi: 10.1016/j.jprot.2013.08.007. Epub 2013 Aug 19. J Proteomics. 2013. PMID: 23969227
References
-
- Campisi J. Replicative senescence: an old lives’ tale? Cell. 1996;84(4):497–500. 10.1016/s0092-8674(00)81023-5. - PubMed
-
- Netterfield TS, Ostheimer GJ, Tentner AR, Joughin BA, Dakoyannis AM, Sharma CD, Sorger PK, Janes KA, Lauffenburger DA, Yaffe MB. Biphasic JNK-Erk signaling separates the induction and maintenance of cell senescence after DNA damage induced by topoisomerase II inhibition. Cell Syst. 2023;14(7):582-604.e510. 10.1016/j.cels.2023.06.005. - PMC - PubMed
-
- Yaswen P, Campisi J. Oncogene-induced senescence pathways weave an intricate tapestry. Cell. 2007;128(2):233–4. 10.1016/j.cell.2007.01.005. - PubMed
-
- Wang Z, Wei D, Xiao H. Methods of cellular senescence induction using oxidative stress. Methods Mol Biol. 2013;1048:135–44. 10.1007/978-1-62703-556-9_11. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
