Role of polyamines in intestinal mucosal barrier function
- PMID: 39836273
- PMCID: PMC11750915
- DOI: 10.1007/s00281-024-01035-4
Role of polyamines in intestinal mucosal barrier function
Abstract
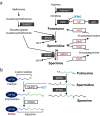
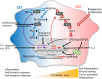
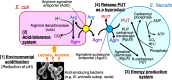
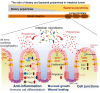
The intestinal epithelium is a rapidly self-renewing tissue; the rapid turnover prevents the invasion of pathogens and harmful components from the intestinal lumen, preventing inflammation and infectious diseases. Intestinal epithelial barrier function depends on the epithelial cell proliferation and junctions, as well as the state of the immune system in the lamina propria. Polyamines, particularly putrescine, spermidine, and spermine, are essential for many cell functions and play a crucial role in mammalian cellular homeostasis, such as that of cell growth, proliferation, differentiation, and maintenance, through multiple biological processes, including translation, transcription, and autophagy. Although the vital role of polyamines in normal intestinal epithelial cell growth and barrier function has been known since the 1980s, recent studies have provided new insights into this topic at the molecular level, such as eukaryotic initiation factor-5A hypusination and autophagy, with rapid advances in polyamine biology in normal cells using biological technologies. This review summarizes recent advances in our understanding of the role of polyamines in regulating normal, non-cancerous, intestinal epithelial barrier function, with a particular focus on intestinal epithelial renewal, cell junctions, and immune cell differentiation in the lamina propria.
Keywords: Cell proliferation; Inflammation; Intestinal microbiome; Intestinal mucosal barrier; Polyamines.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval: No human and animal experiment data were generated for this review article. Competing Interests: The authors declare no competing interests.
Figures

Similar articles
-
Polyamines regulate mitochondrial metabolism essential for intestinal epithelial renewal and wound healing.Am J Physiol Gastrointest Liver Physiol. 2025 Jul 1;329(1):G191-G200. doi: 10.1152/ajpgi.00023.2025. Epub 2025 Jun 5. Am J Physiol Gastrointest Liver Physiol. 2025. PMID: 40471931 Free PMC article.
-
The role of polyamine metabolism in cellular function and physiology.Am J Physiol Cell Physiol. 2024 Aug 1;327(2):C341-C356. doi: 10.1152/ajpcell.00074.2024. Epub 2024 Jun 17. Am J Physiol Cell Physiol. 2024. PMID: 38881422 Free PMC article. Review.
-
The role of the mucosal barrier system in maintaining gut symbiosis to prevent intestinal inflammation.Semin Immunopathol. 2024 Nov 26;47(1):2. doi: 10.1007/s00281-024-01026-5. Semin Immunopathol. 2024. PMID: 39589551 Free PMC article. Review.
-
Polyamines stimulate the protein synthesis of the translation initiation factor eIF5A2, participating in mRNA decoding, distinct from eIF5A1.J Biol Chem. 2025 Aug;301(8):110453. doi: 10.1016/j.jbc.2025.110453. Epub 2025 Jul 4. J Biol Chem. 2025. PMID: 40617352 Free PMC article.
-
Novel Insight into the Multiple Biological Characteristics of Polyamines in the Gut: From Structure to Function.Front Biosci (Landmark Ed). 2025 Jun 30;30(7):27929. doi: 10.31083/FBL27929. Front Biosci (Landmark Ed). 2025. PMID: 40765335 Review.
Cited by
-
Immunomodulatory mechanisms of the gut microbiota and metabolites on regulatory T cells in rheumatoid arthritis.Front Immunol. 2025 Jul 7;16:1610254. doi: 10.3389/fimmu.2025.1610254. eCollection 2025. Front Immunol. 2025. PMID: 40692793 Free PMC article. Review.
-
Therapeutic potential of fecal microbiota transplantation in colorectal cancer based on gut microbiota regulation: from pathogenesis to efficacy.Therap Adv Gastroenterol. 2025 Mar 18;18:17562848251327167. doi: 10.1177/17562848251327167. eCollection 2025. Therap Adv Gastroenterol. 2025. PMID: 40104324 Free PMC article. Review.
References
-
- van der Flier LG, Clevers H (2009) Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol 71:241–260. 10.1146/annurev.physiol.010908.163145 - PubMed
-
- Kim M, Ashida H, Ogawa M, Yoshikawa Y, Mimuro H, Sasakawa C (2010) Bacterial interactions with the host epithelium. Cell Host Microbe 8:20–35. 10.1016/j.chom.2010.06.006 - PubMed
-
- Williams JM, Duckworth CA, Watson AJ, Frey MR, Miguel JC, Burkitt MD, Sutton R, Hughes KR, Hall LJ, Caamano JH, Campbell BJ, Pritchard DM (2013) A mouse model of pathological small intestinal epithelial cell apoptosis and shedding induced by systemic administration of lipopolysaccharide. Dis Model Mech 6:1388–1399. 10.1242/dmm.013284 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

