Safety of Human USH1C Transgene Expression Following Subretinal Injection in Wild-Type Pigs
- PMID: 39836403
- PMCID: PMC11756606
- DOI: 10.1167/iovs.66.1.48
Safety of Human USH1C Transgene Expression Following Subretinal Injection in Wild-Type Pigs
Abstract
Purpose: This study aimed to evaluate early-phase safety of subretinal application of AAVanc80.CAG.USH1Ca1 (OT_USH_101) in wild-type (WT) pigs, examining the effects of a vehicle control, low dose, and high dose.
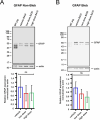
Methods: Twelve WT pigs (24 eyes) were divided into three groups: four pigs each received bilateral subretinal injections of either vehicle, low dose (3.3 × 1010 vector genomes [vg] per eye), or high dose (1.0 × 1011 vg per eye). Total retinal thickness (TRT) was evaluated using optical coherence tomography and retinal function was assessed with full-field electroretinography (ff-ERG) at baseline and two months post-surgery. After necropsy, retinal changes were examined through histopathology, and human USH1C_a1/harmonin expression was assessed by quantitative PCR (qPCR) and Western blotting.
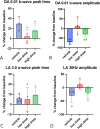
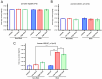
Results: OT_USH_101 led to high USH1C_a1 expression in WT pig retinas without significant TRT changes two months after subretinal injection. The qPCR revealed expression of the human USH1C_a1 transgene delivered by the adeno-associated virus vector. TRT changes were minimal across groups: vehicle (256 ± 21 to 243 ± 18 µm; P = 0.108), low dose (251 ± 32 to 258 ± 30 µm; P = 0.076), and high dose (242 ± 24 to 259 ± 28 µm; P = 0.590). The ff-ERG showed no significant changes in rod or cone responses. Histopathology indicated no severe retinal adverse effects in the vehicle and low dose groups.
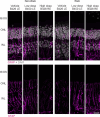
Conclusions: Early-phase clinical imaging, electrophysiology, and histopathological assessments indicated that subretinal administration of OT_USH_101 was well tolerated in the low-dose treatment arm. OT_USH_101 treatment resulted in high expression of human USH1C_a1. Although histopathological changes were not severe, more frequent changes were observed in the high-dose group.
Conflict of interest statement
Disclosure:
Figures







References
-
- Vernon M. Usher's syndrome–deafness and progressive blindness. Clinical cases, prevention, theory and literature survey. J Chronic Dis. 1969; 22: 133–151. - PubMed
-
- Boughman JA, Vernon M, Shaver KA.. Usher syndrome: definition and estimate of prevalence from two high-risk populations. J Chronic Dis. 1983; 36: 595–603. - PubMed
-
- Marazita ML, Ploughman LM, Rawlings B, Remington E, Arnos KS, Nance WE. Genetic epidemiological studies of early-onset deafness in the U.S. school-age population. Am J Med Genet. 1993; 46: 486–491. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

