Outcomes of a Population-Based Congenital Cytomegalovirus Screening Program
- PMID: 39836409
- PMCID: PMC11877178
- DOI: 10.1001/jamapediatrics.2024.5562
Outcomes of a Population-Based Congenital Cytomegalovirus Screening Program
Abstract
Importance: Detection of congenital cytomegalovirus (cCMV) infection has previously relied on targeted screening programs or clinical recognition; however, these approaches miss most cCMV-infected newborns and fail to identify those infants who are asymptomatic at birth but at risk for late-onset sensorineural hearing loss.
Objective: To determine the feasibility of using routinely collected newborn dried blood spots (DBS) in a population-based cCMV screen to identify infants at risk for hearing loss and describe outcomes of infants screened.
Design, setting, and participants: This diagnostic study of a population-based screening program in Ontario, Canada, took place from July 29, 2019, to July 31, 2023. All newborns with a DBS sample collected as part of routine care were screened using polymerase chain reaction (PCR) analysis for cCMV as a risk factor for hearing loss. Infants with positive DBS PCR results for cCMV were referred for confirmation of infection by urine PCR (the gold standard), as well as complete medical and audiologic assessments for sequelae of cCMV infection. Infants with possible or confirmed symptomatic cCMV were referred to pediatric infectious disease specialists for evaluation for potential treatment with valganciclovir.
Exposure: Detection of cCMV by polymerase chain reaction assay on a newborn DBS.
Main outcomes and measures: Number of infants with positive screening results successfully retrieved and confirmed to have cCMV and the timeliness of retrieval and symptomatic evaluation.
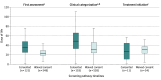
Results: Of 565 987 infants born in the screening period, 551 034 (97.4%) received cCMV screening on the DBS (45.7% female, 54.3% male). Of these infants, 689 (0.13%) screened positive for cCMV; 601 (87.2%) had cCMV infection confirmed and a complete assessment of sequelae of their congenital infection. Ninety-six infants with completed assessments (16.0%) were deemed to have cCMV symptoms, and 63 of these (65.6%) began valganciclovir treatment. Sensorineural hearing loss was confirmed in 34 of 96 infants (35.4%).
Conclusions and relevance: This program found acceptable and feasible implementation of a population-based screening program using routinely collected DBS samples, suggesting that it may serve as a template for jurisdictions considering universal cCMV screening. The program had a much lower than expected prevalence of cCMV-positive screens but still identified many children who would otherwise not have been diagnosed and who would benefit from ongoing audiologic surveillance.
Conflict of interest statement
Figures

Comment on
-
Congenital Cytomegalovirus Screening Moves Ahead.JAMA Pediatr. 2025 Mar 1;179(3):241-243. doi: 10.1001/jamapediatrics.2024.5569. JAMA Pediatr. 2025. PMID: 39836395 No abstract available.

