Map2k6 is a potent genetic modifier of arterial rupture in vascular Ehlers-Danlos syndrome mice
- PMID: 39836470
- PMCID: PMC11949044
- DOI: 10.1172/jci.insight.187315
Map2k6 is a potent genetic modifier of arterial rupture in vascular Ehlers-Danlos syndrome mice
Abstract
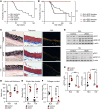
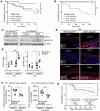
Aortic dissection or rupture is a major cause of mortality in vascular Ehlers-Danlos syndrome (vEDS), a connective tissue disorder caused by heterozygous mutations in the collagen type III alpha 1 chain (COL3A1) gene. C57BL6/J (BL6) mice carrying the Col3a1G938D/+ mutation recapitulate the vEDS vascular phenotype and die suddenly of aortic rupture/dissection. However, 129S6/SvEvTac (referred to here as 129) mice expressing the same Col3a1G938D/+ mutation show near-complete lifelong protection from vascular rupture. To identify genetic modifiers of vascular risk in vEDS, we performed genome-wide genotyping of intercrossed BL6/129 vEDS mice stratified by survival and identified a significant protective locus encompassing a variant in Map2k6, encoding mitogen-activated protein kinase kinase 6 (M2K6), a p38-activating kinase. Genetic ablation of Map2k6 rendered previously protected 129 vEDS mice susceptible to aortic rupture, in association with reduced protein phosphatase 1 activity and increased PKC and ERK phosphorylation. Accelerated vascular rupture in vEDS mice treated with a pharmacological inhibitor of p38 was rescued by concomitant ERK antagonism, supporting an opposing role for ERK and p38 in the modification of aortic rupture risk in vEDS. These results suggest that pharmacologic strategies aimed at mimicking the effect of this natural protective pathway may attenuate aortic rupture risk in vEDS.
Keywords: Cardiology; Collagens; Genetic diseases; Genetic variation; Genetics; Vascular biology.
Conflict of interest statement
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

